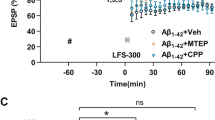
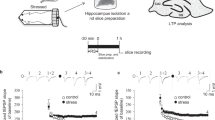
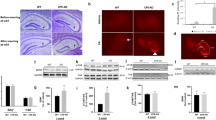
Abstract
Despite considerable progress in the understanding of its neuropathology, Alzheimer’s disease (AD) remains a complex disorder with no effective treatment that counteracts the memory deficits and the underlying synaptic malfunction triggered by the accumulation of amyloid beta (Aβ) and Tau protein. Mounting evidence supports a precipitating role for chronic environmental stress and glutamatergic excitotoxicity in AD, suggesting that targeting of glutamate receptor signaling may be a promising approach against both stress and AD pathologies. In light of the limited cognitive benefit of the direct antagonism of NMDA receptors in AD, we here focus on an alternative way to modify glutamatergic signaling through positive allosteric modulation of AMPA receptors, by the use of a PAM-AMPA compound. Using non-transgenic animal model of Aβ oligomer injection as well as the combined stress and Aβ i.c.v. infusion, we demonstrate that positive allosteric modulation of AMPA receptors by PAM-AMPA treatment reverted memory, but not mood, deficits. Furthermore, PAM-AMPA treatment reverted stress/Aβ-driven synaptic missorting of Tau and associated Fyn/GluN2B-driven excitotoxic synaptic signaling accompanied by recovery of neurotransmitter levels in the hippocampus. Our findings suggest that positive allosteric modulation of AMPA receptors restores synaptic integrity and cognitive performance in stress- and Aβ-evoked hippocampal pathology. As the prevalence of AD is increasing at an alarming rate, novel therapeutic targeting of glutamatergic signaling should be further explored against the early stages of AD synaptic malfunction with the goal of attenuating further synaptic damage before it becomes irreversible.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Goedert M, Spillantini MG. A century of Alzheimer’s disease. Science. 2006;314:777–81.
Duyckaerts C, Delatour B, Potier MC. Classification and basic pathology of Alzheimer disease. Acta Neuropathol. 2009;118:5–36.
Serrano-Pozo A, Frosch MP, Masliah E, Hyman BT. Neuropathological alterations in Alzheimer disease. Cold Spring Harb Perspect Med. 2011;1:1–23.
Koffie RM, Meyer-Luehmann M, Hashimoto T, Adams KW, Mielke ML, Garcia-Alloza M, et al. Oligomeric amyloid beta associates with postsynaptic densities and correlates with excitatory synapse loss near senile plaques. Proc Natl Acad Sci USA. 2009;106:4012–7.
Sperling R, Mormino E, Johnson K. The evolution of preclinical Alzheimer’s disease: Implications for prevention trials. Neuron. 2014;84:608–22.
Scheff SW, Price DA, Schmitt FA, Mufson EJ. Hippocampal synaptic loss in early Alzheimer’s disease and mild cognitive impairment. Neurobiol Aging. 2006;27:1372–84.
Kang HJ, Voleti B, Hajszan T, Rajkowska G, Stockmeier CA, Licznerski P, et al. Decreased expression of synapse-related genes and loss of synapses in major depressive disorder. Nat Med. 2012;18:1413–7.
Popoli M, Yan Z, McEwen B, Sanacora G. The stressed synapse: the impact of stress and glucocorticoids on glutamate transmission. Nat Rev Neurosci. 2011;13:22–37.
Sotiropoulos I, Cerqueira JJ, Catania C, Takashima A, Sousa N, Almeida OFX. Stress and glucocorticoid footprints in the brain—the path from depression to Alzheimer’s disease. Neurosci Biobehav Rev. 2008;32:1161–73.
Elgh E, Lindqvist Astot A, Fagerlund M, Eriksson S, Olsson T, Näsman B. Cognitive dysfunction, hippocampal atrophy and glucocorticoid feedback in Alzheimer’s disease. Biol Psychiatry. 2006;59:155–61.
Simard M, Hudon C, van Reekum R. Psychological distress and risk for dementia. Curr Psychiatry Rep. 2009;11:41–7.
Green KN, Billings LM, Roozendaal B, McGaugh JL, LaFerla FM. Glucocorticoids increase amyloid-β and Tau pathology in a mouse model of Alzheimer’s Disease. J Neurosci. 2006;26:9047–56.
Jeong YH, Park CH, Yoo J, Shin KY, Ahn S-M, Kim H-S, et al. Chronic stress accelerates learning and memory impairments and increases amyloid deposition in APPV717I-CT100 transgenic mice, an Alzheimer’s disease model. FASEB J. 2006;20:729–31.
Sotiropoulos I, Catania C, Pinto LG, Silva R, Pollerberg GE, Takashima A, et al. Stress acts cumulatively to precipitate Alzheimer’s disease-like Tau pathology and cognitive deficits. J Neurosci. 2011;31:7840–7.
Silva JM, Rodrigues S, Sampaio-Marques B, Gomes P, Neves-Carvalho A, Dioli C, et al. Dysregulation of autophagy and stress granule-related proteins in stress-driven Tau pathology. Cell Death Differ. 2019;26:1411–27.
Rudy CC, Hunsberger HC, Weitzner DS, Reed MN. The role of the tripartite glutamatergic synapse in the pathophysiology of Alzheimer’s disease. Aging Dis. 2015;6:131–48.
Henley JM, Wilkinson KA. AMPA receptor trafficking and the mechanisms underlying synaptic plasticity and cognitive aging. Dialogues Clin Neurosci. 2013;15:11–27.
Lesuis SL, Lucassen PJ, Krugers HJ. Early life stress impairs fear memory and synaptic plasticity; a potential role for GluN2B. Neuropharmacol. 2019;149:195–203.
Jacob CP, Koutsilieri E, Bartl J, Neuen-Jacob E, Arzberger T, Zander N, et al. Alterations in expression of glutamatergic transporters and receptors in sporadic Alzheimer’s disease. JAD. 2007;11:97–116.
Chapman PF, White GL, Jones MW, Cooper-Blacketer D, Marshall VJ, Irizarry M, et al. Impaired synaptic plasticity and learning in aged amyloid precursor protein transgenic mice. Nat Neurosci. 1999;2:271–6.
Hsieh H, Boehm J, Sato C, Iwatsubo T, Tomita T, Sisodia S, et al. AMPAR removal underlies abeta-induced synaptic depression and dendritic spine loss. Neuron. 2006;52:831–43.
Shankar GM, Bloodgood BL, Townsend M, Walsh DM, Selkoe DJ, Sabatini BL. Natural oligomers of the Alzheimer amyloid-protein induce reversible synapse loss by modulating an NMDA-Type glutamate receptor-dependent signaling pathway. J Neurosci. 2007;27:2866–75.
Wu H-Y, Hudry E, Hashimoto T, Kuchibhotla K, Rozkalne A, Fan Z, et al. Amyloid beta induces the morphological neurodegenerative triad of spine loss, dendritic simplification, and neuritic dystrophies through calcineurin activation. J Neurosci. 2010;30:2636–49.
Zempel H, Thies E, Mandelkow E, Mandelkow E-M. A oligomers cause localized Ca2+ elevation, missorting of endogenous Tau into dendrites, Tau phosphorylation, and destruction of microtubules and spines. J Neurosci. 2010;30:11938–50.
Ittner LM, Ke YD, Delerue F, Bi M, Gladbach A, van Eersel J, et al. Dendritic function of tau mediates amyloid-beta toxicity in Alzheimer’s disease mouse models. Cell. 2010;142:387–97.
Sotiropoulos I, Sousa N. Tau as the converging protein between chronic stress and Alzheimer’s disease synaptic pathology. Neurodegener Dis. 2016;16:22–5.
Yang C-H, Huang C-C, Hsu K-S. Behavioral stress enhances hippocampal CA1 long-term depression through the blockade of the glutamate uptake. J Neurosci. 2005;25:4288–93.
Lesuis SL, Kaplick PM, Lucassen PJ, Krugers HJ. Treatment with the glutamate modulator riluzole prevents early life stress-induced cognitive deficits and impairments in synaptic plasticity in APPswe/PS1dE9 mice. Neuropharmacol. 2019;150:175–83.
Lopes S, Vaz-Silva J, Pinto V, Dalla C, Kokras N, Bedenk B, et al. Tau protein is essential for stress-induced brain pathology. Proc Nat Acad Sci USA. 2016;113:E3755–63.
Pinheiro S, Silva J, Mota C, Vaz-Silva J, Veloso A, Pinto V, et al. Tau mislocation in glucocorticoid-triggered hippocampal pathology. Mol Neurobiol. 2016;53:4745–53.
Prediger RDS, Franco JL, Pandolfo P, Medeiros R, Duarte FS, Di Giunta G, et al. Differential susceptibility following β-amyloid peptide-(1-40) administration in C57BL/6 and Swiss albino mice: evidence for a dissociation between cognitive deficits and the glutathione system response. Behav. Brain Res. 2007;177:205–13.
Catania C, Sotiropoulos I, Silva R, Onofri C, Breen KC, Sousa N, et al. The amyloidogenic potential and behavioral correlates of stress. Mol Psychiatry. 2009;14:95–105.
Graziano A, Petrosini L, Bartoletti A. Automatic recognition of explorative strategies in the Morris water maze. J Neurosci Methods. 2003;130:33–44.
Hoover BR, Reed MN, Su J, Penrod RD, Kotilinek LA, Grant MK, et al. Tau mislocalization to dendritic spines mediates synaptic dysfunction independently of neurodegeneration. Neuron. 2010;68:1067–81.
Salter MW, Kalia LV. Src kinases: a hub for NMDA receptor regulation. Nat Rev Neurosci. 2004;5:317–28.
Cummings J, Lee G, Ritter A, Zhong K. Alzheimer’s disease drug development pipeline: 2018. Alzheimers Dement (N. Y). 2018;4:195–214.
Vaz‐Silva J, Gomes P, Jin Q, Zhu M, Zhuravleva V, Quintremil S, et al. Endolysosomal degradation of Tau and its role in glucocorticoid‐driven hippocampal malfunction. EMBO J. 2018;37:e99084.
Sotiropoulos I, Silva JMGM, Kimura T, Rodrigues AJ, Costa PS, Almeida OFX, et al. Female hippocampus vulnerability to environmental stress, a precipitating factor in tau aggregation pathology. J Alzheimers Dis. 2015;43:763–74.
Panza F, Lozupone M, Logroscino G, Imbimbo BP. A critical appraisal of amyloid-β-targeting therapies for Alzheimer disease. Nat Rev Neurol. 2019;15:73–88.
Iaccarino HF, Singer AC, Martorell AJ, Rudenko A, Gao F, Gillingham TZ, et al. Gamma frequency entrainment attenuates amyloid load and modifies microglia. Nature. 2016;540:230–5.
Griñán-Ferré C, Izquierdo V, Otero E, Puigoriol-Illamola D, Corpas R, Sanfeliu C, et al. Environmental enrichment improves cognitive deficits, AD hallmarks and epigenetic alterations presented in 5xFAD mouse model. Front Cell Neurosci Front Cell Neurosci. 2018;12:224.
Weiner MW, Sadowsky C, Saxton J, Hofbauer RK, Graham SM, Yun S, et al. Magnetic resonance imaging and neuropsychological results from a trial of memantine in Alzheimer’ s disease. Alzheimer’s Dement. 2011;7:425–35.
Kumar J, Mayer ML. Functional insights from glutamate receptor ion channel structures. Annu Rev Physiol. 2013;75:313–37.
Lynch G, Gall CM. Ampakines and the threefold path to cognitive enhancement. Trends Neurosci. 2006;29:554–62.
Black MD. Therapeutic potential of positive AMPA modulators and their relationship to AMPA receptor subunits. A review of preclinical data. Psychopharmacol. 2005;4:154–63.
Bernard K, Danober L, Thomas J, Mu C, Cordi A, Desos P, et al. S 18986: A positive allosteric modulator of AMPA-type glutamate receptors pharmacological profile of a novel cognitive enhancer. CNS Neurosc Ther. 2010;16:193–212.
Bretin S, Louis C, Seguin L, Wagner S, Thomas J-Y, Challal S, et al. Pharmacological characterisation of S 47445, a novel positive allosteric modulator of AMPA receptors. PLoS One. 2017;12:e0184429.
Giralt A, Gómez-Climent MÁ, Alcalá R, Bretin S, Bertrand D, María Delgado-García J, et al. The AMPA receptor positive allosteric modulator S 47445 rescues in vivo CA3-CA1 long-term potentiation and structural synaptic changes in old mice. Neuropharmacology. 2017;123:395–409.
Dolan RJ. Emotion, cognition, and behavior. Science. 2002;298:1191–4.
Grossberg GT, Desai AK. Management of Alzheimer’s Disease. J Gerontol A Biol Sci Med Sci. 2003;58:M331–53.
Refojo D, Schweizer M, Kuehne C, Ehrenberg S, Thoeringer C, Vogl AM, et al. Glutamatergic and dopaminergic neurons mediate anxiogenic and anxiolytic effects of CRHR1. Science. 2011;333:1903–7.
Mossello E, Boncinelli M, Caleri V, Cavallini MC, Palermo E, Di Bari M, et al. Is antidepressant treatment associated with reduced cognitive decline in Alzheimer’s Disease? Dement Geriatr Cogn Disord. 2008;25:372–9.
Jaworski T, Lechat B, Demedts D, Gielis L, Devijver H, Borghgraef P, et al. Dendritic degeneration, neurovascular defects, and inflammation precede neuronal loss in a mouse model for tau-mediated neurodegeneration. Am J Pathol. 2011;179:2001–15.
Decker H, Jürgensen S, Adrover MF, Brito-Moreira J, Bomfim TR, Klein WL, et al. N-methyl-D-aspartate receptors are required for synaptic targeting of Alzheimer’s toxic amyloid-β peptide oligomers. J Neurochem. 2010;115:1520–9.
Miyamoto T, Kim D, Knox JA, Johnson E, Mucke L. Increasing the receptor tyrosine kinase EphB2 prevents amyloid-β-induced depletion of cell surface glutamate receptors by a mechanism that requires the PDZ-binding motif of EphB2 and neuronal activity. J Biol Chem. 2016;291:1719–34. 22
Baglietto‐Vargas D, Prieto GA, Limon A, Forner S, Rodriguez‐Ortiz CJ, Ikemura K, et al. Impaired AMPA signaling and cytoskeletal alterations induce early synaptic dysfunction in a mouse model of Alzheimer’s disease. Aging Cell. 2018;17:e12791.
Tanaka H, Sakaguchi D, Hirano T. Amyloid-β oligomers suppress subunit-specific glutamate receptor increase during LTP. Alzheimer’s Dement. 2019;5:797–808.
Reinders NR, Pao Y, Renner MC, Silva-Matos CM, da, Lodder TR, Malinow R, et al. Amyloid-β effects on synapses and memory require AMPA receptor subunit GluA3. PNAS. 2016;113:E6526–34.
Berchtold NC, Sabbagh MN, Beach TG, Kim RC, Cribbs DH, Cotman CW. Brain gene expression patterns differentiate mild cognitive impairment from normal aged and Alzheimer disease. Neurobiol Aging. 2014;35:1961–72.
Tai H-C, Serrano-Pozo A, Hashimoto T, Frosch MP, Spires-Jones TL, Hyman BT. The synaptic accumulation of hyperphosphorylated tau oligomers in Alzheimer disease is associated with dysfunction of the ubiquitin-proteasome system. Am J Pathol. 2012;181:1426–35.
Zhou L, McInnes J, Wierda K, Holt M, Herrmann AG, Jackson RJ, et al. Tau association with synaptic vesicles causes presynaptic dysfunction. Nat Commun. 2017;8:1–13.
McInnes J, Wierda K, Snellinx A, Bounti L, Wang Y-C, Stancu I-C, et al. Synaptogyrin-3 mediates presynaptic dysfunction induced by Tau. Neuron. 2018;97:823–835.e8.
Borodovitsyna O, Flamini M, Chandler D. Noradrenergic modulation of cognition in health and disease. Neural Plast. 2017; 6031478. https://doi.org/10.1155/2017/6031478.
Švob Štrac D, Pivac N, Mück-Šeler D. The serotonergic system and cognitive function. Transl Neurosci. 2016;7:35–49.
Zhang L, Ouyang M, Ganellin CR, Thomas SA. The slow afterhyperpolarization: a target of β1-adrenergic signaling in hippocampus-dependent memory retrieval. J Neurosci. 2013;33:5006–16.
Lai MKP, Tsang SWY, Francis PT, Keene J, Hope T, Esiri MM, et al. Postmortem serotoninergic correlates of cognitive decline in Alzheimer’s disease. Neuroreport. 2002;13:1175–8.
Calabrese F, Savino E, Mocaer E, Bretin S, Racagni G, Riva MA. Upregulation of neurotrophins by S 47445, a novel positive allosteric modulator of AMPA receptors in aged rats. Pharm Res. 2017;121:59–69.
Giralt A, Gómez-climent MÁ, Alcalá R, Bretin S, Delgado-garcía JM, Pérez-navarro E, et al. The AMPA receptor positive allosteric modulator S 47445 rescues in vivo CA3-CA1 long-term potentiation and structural synaptic changes in old mice. Neuropharmacolgy. 2017;123:395–409.
Holmes C, Boche D, Wilkinson D, Yadegarfar G, Hopkins V, Bayer A, et al. Long-term effects of abeta42 immunisation in Alzheimer’s disease: follow-up of a randomised, placebo-controlled phase I trial. Lancet. 2008;372:216–23.
Bernard K, Gouttefangeas S, Bretin S, Galtier S, Robert P, Holthoff-Detto V, et al. A 24-week double-blind placebo-controlled study of the efficacy and safety of the AMPA modulator S47445 in patients with mild to moderate Alzheimer’s disease and depressive symptoms. Alzheimers Dement (N. Y). 2019;5:231–40.
Acknowledgements
We thank Drs Luisa Pinto and Patricia Patricio (BnML & ICVS, School of Medicine, University of Minho, Portugal) for their administrative support. In addition, we would like to thank Dr Osborne F.X. Almeida (Max Planck Institute of Psychiatry, Germany) for his critical and constructive comments and feedback on the paper. This work was funded by the Institut de Recherches Internationales Servier (France). Carina Soares-Cunha is holder of a post-doctoral fellowship from the Programa de Atividades Conjuntas (PAC), through MEDPERSYST project (POCI-01-0145-FEDER-016428), supported by the Portugal 2020 Programme Fund (FEDER) while these studies were also supported by the European Regional Development Fund COMPETE (FCOMP-01-0124-FEDER-037298) as well as the project NORTE-01-0145-FEDER-000013, supported by the Northern Portugal Regional Operational Programme (NORTE 2020), under the Portugal 2020 Partnership Agreement, through the European Regional Development.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
This study was partly financially supported by the Institut de Recherches Internationales Servier (France) which has no influence on the experimental performance, tissue collection, and analysis as well as data analysis. SB and FA and RB are employees of Institut de Recherches Internationales Servier.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Monteiro-Fernandes, D., Silva, J.M., Soares-Cunha, C. et al. Allosteric modulation of AMPA receptors counteracts Tau-related excitotoxic synaptic signaling and memory deficits in stress- and Aβ-evoked hippocampal pathology. Mol Psychiatry 26, 5899–5911 (2021). https://doi.org/10.1038/s41380-020-0794-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41380-020-0794-5
This article is cited by
-
Advances in Molecular Psychiatry – March 2023: mitochondrial function, stress, neuroinflammation – bipolar disorder, psychosis, and Alzheimer’s disease
Molecular Psychiatry (2023)
-
Research Progress on Exosomes and MicroRNAs in the Microenvironment of Postoperative Neurocognitive Disorders
Neurochemical Research (2022)
-
Molecular Psychiatry special issue: advances in Alzheimer’s disease
Molecular Psychiatry (2021)