Abstract
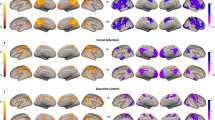
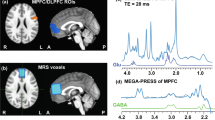
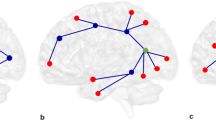
Psychotic Disorders such as schizophrenia (SZ) and bipolar disorder (BD) are characterized by abnormal functional connectivity (FC) within neural networks such as the default mode network (DMN), as well as attenuated anticorrelation between DMN and task-positive networks (TPN). Bioenergetic processes are critical for synaptic connectivity and are also abnormal in psychotic disorders. We therefore examined the association between brain energy metabolism and FC in psychotic disorders. 31P magnetization transfer spectroscopy from medial prefrontal cortex (MPFC) and whole-brain fMRI data were collected from demographically matched groups of SZ, BD, and healthy control (HC) subjects. The creatine kinase (CK) reaction flux calculated from spectroscopy was used as an index of regional energy production rate. FC maps were generated with MPFC as the seed region. Compared to HC, SZ showed significantly lower CK flux, while both BD and SZ patients showed decreased anticorrelation between MPFC and TPN. CK flux was significantly correlated with FC between MPFC and other DMN nodes in HC. This positive correlation was reduced modestly in BD and strongly in SZ. CK flux was negatively correlated with the anticorrelation between MPFC and TPN in HC, but this relationship was not observed in BD or SZ. These results indicate that MPFC energy metabolism rates are associated with stronger FC within networks and stronger anticorrelation between networks in HC. However, this association is decreased in SZ and BD, where bioenergetic and FC abnormalities are evident. This pattern may suggest that impairment in energy production in psychotic disorders underlies the impaired neural connectivity.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Friston K, Brown HR, Siemerkus J, Stephan KE. The dysconnection hypothesis (2016). Schizophrenia Res. 2016;176:83–94.
Whitfield-Gabrieli S, Thermenos HW, Milanovic S, Tsuang MT, Faraone SV, McCarley RW, et al. Hyperactivity and hyperconnectivity of the default network in schizophrenia and in first-degree relatives of persons with schizophrenia. Proc Natl Acad Sci. 2009;106:1279–84.
Hu ML, Zong XF, Mann JJ, Zheng JJ, Liao YH, Li ZC, et al. A Review of the Functional and Anatomical Default Mode Network in Schizophrenia. Neurosci Bull. 2017;33:73–84.
Du F, Cooper AJ, Thida T, Sehovic S, Lukas SE, Cohen BM, et al. In Vivo Evidence for Cerebral Bioenergetic Abnormalities in Schizophrenia Measured Using 31P Magnetization Transfer SpectroscopySchizophrenia Cerebral Bioenergetic AbnormalitiesSchizophrenia Cerebral Bioenergetic Abnormalities. JAMA Psychiatry. 2014;71:19–27.
Du F, Yuksel C, Chouinard VA, Huynh P, Ryan K, Cohen BM, et al. Abnormalities in High-Energy Phosphate Metabolism in First-Episode Bipolar Disorder Measured Using (31)P-Magnetic Resonance Spectroscopy. Biol Psychiatry. 2018;84:797–802.
Du F, Zhu XH, Qiao H, Zhang X, Chen W. Efficient in vivo 31P magnetization transfer approach for noninvasively determining multiple kinetic parameters and metabolic fluxes of ATP metabolism in the human brain. Magn Reson Med. 2007;57:103–14.
Logothetis NK. The underpinnings of the BOLD functional magnetic resonance imaging signal. J Neurosci. 2003;23:3963–71.
de Graaf RA, Luo Y, Garwood M, Nicolay K. B1-Insensitive, Single-Shot Localization and Water Suppression. J Magn Reson, Ser B. 1996;113:35–45.
Du F, Zhu XH, Zhang Y, Friedman M, Zhang N, Ugurbil K, et al. Tightly coupled brain activity and cerebral ATP metabolic rate. Proc Natl Acad Sci USA. 2008;105:6409–14.
Murphy K, Fox MD. Towards a consensus regarding global signal regression for resting state functional connectivity MRI. NeuroImage. 2017;154:169–73.
Liu TT, Nalci A, Falahpour M. The global signal in fMRI: nuisance or Information? NeuroImage. 2017;150:213–29.
Song X, Zhang Y, Liu Y. Frequency specificity of regional homogeneity in the resting-state human brain. PloS ONE. 2014;9:e86818.
Zang Y, Jiang T, Lu Y, He Y, Tian L. Regional homogeneity approach to fMRI data analysis. NeuroImage. 2004;22:394–400.
Zou Q-H, Zhu C-Z, Yang Y, Zuo X-N, Long X-Y, Cao Q-J, et al. An improved approach to detection of amplitude of low-frequency fluctuation (ALFF) for resting-state fMRI: fractional ALFF. J Neurosci Methods. 2008;172:137–41.
Song X, Qian S, Liu K, Zhou S, Zhu H, Zou Q, et al. Resting-state BOLD oscillation frequency predicts vigilance task performance at both normal and high environmental temperatures. Brain Struct Funct. 2017;222:4065–77.
Basser PJ, Mattiello J, LeBihan D. Estimation of the effective self-diffusion tensor from the NMR spin echo. J Magn Reson Ser B. 1994;103:247–54.
Simpson R, Devenyi GA, Jezzard P, Hennessy TJ, Near J. Advanced processing and simulation of MRS data using the FID appliance (FID-A)-An open source, MATLAB-based toolkit. Magn Reson Med. 2017;77:23–33.
Hetherington HP, Spencer DD, Vaughan JT, Pan JW. Quantitative (31)P spectroscopic imaging of human brain at 4 Tesla: assessment of gray and white matter differences of phosphocreatine and ATP. Magn Reson Med. 2001;45:46–52.
Mori S, Oishi K, Jiang H, Jiang L, Li X, Akhter K, et al. Stereotaxic white matter atlas based on diffusion tensor imaging in an ICBM template. NeuroImage. 2008;40:570–82.
Burbaeva G, Savushkina OK, Boksha IS. Creatine kinase BB in brain in schizophrenia. World J Biol Psychiatry. 2003;4:177–83.
Klushnik TP, Spunde A, Yakovlev AG, Khuchua ZA, Saks VA, Vartanyan ME. Intracellular alterations of the creatine kinase isoforms in brains of schizophrenic patients. Mol Chem Neuropathol. 1991;15:271–80.
Guzun R, Timohhina N, Tepp K, Monge C, Kaambre T, Sikk P, et al. Regulation of respiration controlled by mitochondrial creatine kinase in permeabilized cardiac cells in situ. Importance of system level properties. Biochim Biophys Acta. 2009;1787:1089–105.
Schlattner U, Tokarska-Schlattner M, Wallimann T. Mitochondrial creatine kinase in human health and disease. Biochim Biophys Acta. 2006;1762:164–80.
Hazlett EA, Vaccaro DH, Haznedar MM, Goldstein KE. (F-18)Fluorodeoxyglucose positron emission tomography studies of the schizophrenia spectrum: the legacy of Monte S. Buchsbaum, M.D. Psychiatry Res. 2019;271:535–40.
Williamson P. Are anticorrelated networks in the brain relevant to schizophrenia? Schizophrenia Bull. 2007;33:994–1003.
Wotruba D, Michels L, Buechler R, Metzler S, Theodoridou A, Gerstenberg M, et al. Aberrant coupling within and across the default mode, task-positive, and salience network in subjects at risk for psychosis. Schizophrenia Bull. 2014;40:1095–104.
Fox MD, Zhang D, Snyder AZ, Raichle ME. The global signal and observed anticorrelated resting state brain networks. J Neurophysiol. 2009;101:3270–83.
Fukunaga M, Horovitz SG, van Gelderen P, de Zwart JA, Jansma JM, Ikonomidou VN, et al. Large-amplitude, spatially correlated fluctuations in BOLD fMRI signals during extended rest and early sleep stages. Magn Reson imaging. 2006;24:979–92.
Kiviniemi VJ, Haanpää H, Kantola J-H, Jauhiainen J, Vainionpää V, Alahuhta S, et al. Midazolam sedation increases fluctuation and synchrony of the resting brain BOLD signal. Magn Reson imaging. 2005;23:531–7.
Licata SC, Nickerson LD, Lowen SB, Trksak GH, MacLean RR, Lukas SE. The hypnotic zolpidem increases the synchrony of BOLD signal fluctuations in widespread brain networks during a resting paradigm. NeuroImage. 2013;70:211–22.
Wong CW, Olafsson V, Tal O, Liu TT. The amplitude of the resting-state fMRI global signal is related to EEG vigilance measures. NeuroImage. 2013;83:983–90.
Yang GJ, Murray JD, Repovs G, Cole MW, Savic A, Glasser MF, et al. Altered global brain signal in schizophrenia. Proc Natl Acad Sci. 2014;111:7438–43.
Jevtic G, Nikolic T, Mircic A, Stojkovic T, Velimirovic M, Trajkovic V, et al. Mitochondrial impairment, apoptosis and autophagy in a rat brain as immediate and long-term effects of perinatal phencyclidine treatment - influence of restraint stress. Prog Neuropsychopharmacol Biol Psychiatry. 2016;66:87–96.
Yuksel C, Du F, Ravichandran C, Goldbach JR, Thida T, Lin P, et al. Abnormal high-energy phosphate molecule metabolism during regional brain activation in patients with bipolar disorder. Mol Psychiatry. 2015;20:1079–84.
Chen W, Zhu XH, Adriany G, Ugurbil K. Increase of creatine kinase activity in the visual cortex of human brain during visual stimulation: a 31P magnetization transfer study. Magn Reson Med. 1997;38:551–7.
Tomasi D, Wang GJ, Volkow ND. Energetic cost of brain functional connectivity. Proc Natl Acad Sci USA. 2013;110:13642–7.
Attwell D, Laughlin SB. An energy budget for signaling in the grey matter of the brain. J Cereb Blood Flow Metab. 2001;21:1133–45.
Du F, Ongur D. Probing myelin and axon abnormalities separately in psychiatric disorders using MRI techniques. Front Integr Neurosci. 2013;7:24.
Scheel M, Prokscha T, Bayerl M, Gallinat J, Montag C. Myelination deficits in schizophrenia: evidence from diffusion tensor imaging. Brain Struct Funct. 2013;218:151–6.
Kumar J, Iwabuchi S, Oowise S, Balain V, Palaniyappan L, Liddle PF. Shared white-matter dysconnectivity in schizophrenia and bipolar disorder with psychosis. Psychological Med. 2015;45:759–70.
Mamah D, Ji A, Rutlin J, Shimony JS. White matter integrity in schizophrenia and bipolar disorder: tract- and voxel-based analyses of diffusion data from the Connectom scanner. NeuroImage Clin. 2019;21:101649.
Boos HB, Mandl RC, van Haren NE, Cahn W, van Baal GC, Kahn RS, et al. Tract-based diffusion tensor imaging in patients with schizophrenia and their non-psychotic siblings. Eur Neuropsychopharmacol. 2013;23:295–304.
Clark K, Narr KL, O’Neill J, Levitt J, Siddarth P, Phillips O, et al. White matter integrity, language, and childhood onset schizophrenia. Schizophrenia Res. 2012;138:150–6.
Bracht T, Viher PV, Stegmayer K, Strik W, Federspiel A, Wiest R, et al. Increased structural connectivity of the medial forebrain bundle in schizophrenia spectrum disorders is associated with delusions of paranoid threat and grandiosity. NeuroImage Clin. 2019;24:102044.
Kubicki M, McCarley R, Westin CF, Park HJ, Maier S, Kikinis R, et al. A review of diffusion tensor imaging studies in schizophrenia. J Psychiatr Res. 2007;41:15–30.
Degani H, Laughlin M, Campbell S, Shulman RG. Kinetics of creatine kinase in heart: a 31P NMR saturation- and inversion-transfer study. Biochemistry. 1985;24:5510–6.
Kim SY, Chen W, Ongur D, Du F. Rapid and simultaneous measurement of phosphorus metabolite pool size ratio and reaction kinetics of enzymes in vivo. J Magn Reson Imaging. 2018;47:210–21.
Zhu XH, Qiao H, Du F, Xiong Q, Liu X, Zhang X, et al. Quantitative imaging of energy expenditure in human brain. NeuroImage. 2012;60:2107–17.
Acknowledgements
The authors thank our volunteers and Mr Elliot Kuan, and Ms Margaret Gardner for their assistance in the experiments and subject recruitment. This research work was supported by National Institutes of Health (NIH) grants: R21MH114020, R01MH114982, P50MH115846, K24MH104449, R01AG066670.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Over the past 3 years, Dr. DAP has received consulting fees from Akili Interactive Labs, BlackThorn Therapeutics, Boehringer Ingelheim, Compass Pathway, Otsuka Pharmaceuticals, and Takeda Pharmaceuticals; one honorarium from Alkermes, and research funding from NIMH, Dana Foundation, Brain and Behavior Research Foundation, Millennium Pharmaceuticals. In addition, he has received stock options from BlackThorn Therapeutics. Dr. BF has received research funding from the NIA, Rogers Family Foundation, Spier Family Foundation, Eli Lilly and Biogen and consulting fees from Biogen. Dr. CY received research support from Diamentis Inc. No funding from these entities was used to support the current work, and all views expressed are solely those of the authors. None of the other authors have conflict of interest to declare.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Song, X., Chen, X., Yuksel, C. et al. Bioenergetics and abnormal functional connectivity in psychotic disorders. Mol Psychiatry 26, 2483–2492 (2021). https://doi.org/10.1038/s41380-020-00993-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41380-020-00993-z
This article is cited by
-
Focusing on mitochondria in the brain: from biology to therapeutics
Translational Neurodegeneration (2024)
-
Inversed Effects of Nav1.2 Deficiency at Medial Prefrontal Cortex and Ventral Tegmental Area for Prepulse Inhibition in Acoustic Startle Response
Molecular Neurobiology (2024)
-
Effects of antipsychotic medication on functional connectivity in major depressive disorder with psychotic features
Molecular Psychiatry (2023)
-
Association of default-mode network neurotransmitters and inter-network functional connectivity in first episode psychosis
Neuropsychopharmacology (2023)
-
Magnetic Resonance Spectroscopy Studies of Brain Energy Metabolism in Schizophrenia: Progression from Prodrome to Chronic Psychosis
Current Psychiatry Reports (2023)