Abstract
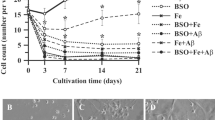
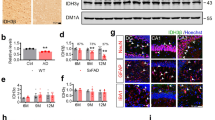
Dysregulation of formaldehyde (FA) has been implicated in the development of Alzheimer’s Disease (AD). Elevated FA levels in Alzheimer’s patients and animal models are associated with impaired cognitive functions. However, the exact role of FA in AD remains unknown. We now identified that oxidative demethylation at serine8/26 of amyloid-beta protein (Aβ) induced FA generation and FA cross-linked with the lysine28 residue in the β-turn of Aβ monomer to form Aβ dimers, and then accelerated Aβ oligomerization and fibrillogenesis in vitro. However, Aβ42 mutation in serine8/26, lysine28 abolished Aβ self-aggregation. Furthermore, Aβ inhibited the activity of formaldehyde dehydrogenase (FDH), the enzyme for FA degradation, resulting in FA accumulation. In turn, excess of FA stimulated Aβ aggregation both in vitro and in vivo by increasing the formation of Aβ oligomers and fibrils. We found that degradation of FA by formaldehyde scavenger-NaHSO3 or coenzyme Q10 reduced Aβ aggregation and ameliorated the neurotoxicity, and improved the cognitive performance in APP/PS1 mice. Our study provides evidence that endogenous FA is essential for Aβ self-aggregation and scavenging FA could be an effective strategy for treating AD.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
Sun X, Bromley-Brits K, Song W. Regulation of beta-site APP-cleaving enzyme 1 gene expression and its role in Alzheimer’s disease. J Neurochem. 2012;120:62–70.
Deng Y, Wang Z, Wang R, Zhang X, Zhang S, Wu Y, et al. Amyloid-beta protein (Abeta) Glu11 is the major beta-secretase site of beta-site amyloid-beta precursor protein-cleaving enzyme 1(BACE1), and shifting the cleavage site to Abeta Asp1 contributes to Alzheimer pathogenesis. Eur J Neurosci. 2013;37:1962–9.
Ly PT, Wu Y, Zou H, Wang R, Zhou W, Kinoshita A, et al. Inhibition of GSK3beta-mediated BACE1 expression reduces Alzheimer-associated phenotypes. J Clin Invest. 2013;123:224–35.
Zhang S, Cai F, Wu Y, Bozorgmehr T, Wang Z, Zhang S, et al. A presenilin-1 mutation causes Alzheimer disease without affecting Notch signaling. Mol Psychiatry. 2020;25:603–13.
Zhang S, Wang Z, Cai F, Zhang M, Wu Y, Zhang J, et al. BACE1 cleavage site selection critical for amyloidogenesis and alzheimer’s pathogenesis. J Neurosci. 2017;37:6915–25.
Hardy JA, Higgins GA. Alzheimer’s disease: the amyloid cascade hypothesis. Science. 1992;256:184–5.
Zhang Y, Song W. Islet amyloid polypeptide: another key molecule in Alzheimer’s pathogenesis? Prog Neurobiol. 2017;153:100–20.
Zott B, Simon MM, Hong W, Unger F, Chen-Engerer HJ, Frosch MP, et al. A vicious cycle of beta amyloid-dependent neuronal hyperactivation. Science. 2019;365:559–65.
Shankar GM, Li S, Mehta TH, Garcia-Munoz A, Shepardson NE, Smith I, et al. Amyloid-beta protein dimers isolated directly from Alzheimer’s brains impair synaptic plasticity and memory. Nat Med. 2008;14:837–42.
Lesne SE. Toxic oligomer species of amyloid-beta in Alzheimer’s disease, a timing issue. Swiss Med Wkly. 2014;144:w14021.
Zhang M, Hausmann L, Song W. Targeting nascent soluble Abeta42 for potential Alzheimer drug development. J Neurochem. 2013;125:329–31.
Zhang S, Zhao J, Zhang Y, Zhang Y, Cai F, Wang L, et al. Upregulation of MIF as a defense mechanism and a biomarker of Alzheimer’s disease. Alzheimers Res Ther. 2019;11:54.
Ahmed M, Davis J, Aucoin D, Sato T, Ahuja S, Aimoto S, et al. Structural conversion of neurotoxic amyloid-beta(1-42) oligomers to fibrils. Nat Struct Mol Biol. 2010;17:561–7.
Bernstein SL, Dupuis NF, Lazo ND, Wyttenbach T, Condron MM, Bitan G, et al. Amyloid-beta protein oligomerization and the importance of tetramers and dodecamers in the aetiology of Alzheimer’s disease. Nat Chem. 2009;1:326–31.
Du Y, Du Y, Zhang Y, Huang Z, Fu M, Li J, et al. MKP-1 reduces Abeta generation and alleviates cognitive impairments in Alzheimer’s disease models. Signal Transduct Target Ther. 2019;4:58.
Liao X, Cai F, Sun Z, Zhang Y, Wang J, Jiao B, et al. Identification of Alzheimer’s disease-associated rare coding variants in the ECE2 gene. JCI Insight. 2020;5:e135119.
Qing H, He G, Ly PT, Fox CJ, Staufenbiel M, Cai F, et al. Valproic acid inhibits Abeta production, neuritic plaque formation, and behavioral deficits in Alzheimer’s disease mouse models. J Exp Med. 2008;205:2781–9.
Sun X, He G, Qing H, Zhou W, Dobie F, Cai F, et al. Hypoxia facilitates Alzheimer’s disease pathogenesis by up-regulating BACE1 gene expression. Proc Natl Acad Sci USA. 2006;103:18727–32.
Rozga M, Bal W. The Cu(II)/Abeta/human serum albumin model of control mechanism for copper-related amyloid neurotoxicity. Chem Res Toxicol. 2010;23:298–308.
Li Y, Zhang L, Wang W. Formation of aldehyde and ketone compounds during production and storage of milk powder. Molecules. 2012;17:9900–11.
Kalasz H. Biological role of formaldehyde, and cycles related to methylation, demethylation, and formaldehyde production. Mini Rev Med Chem. 2003;3:175–92.
Yue X, Mei Y, Zhang Y, Tong Z, Cui D, Yang J, et al. New insight into Alzheimer’s disease: Light reverses Abeta-obstructed interstitial fluid flow and ameliorates memory decline in APP/PS1 mice. Alzheimers Dement (N. Y). 2019;5:671–84.
Tang Y, Kong X, Xu A, Dong B, Lin W. Development of a two-photon fluorescent probe for imaging of endogenous formaldehyde in living tissues. Angew Chem Int Ed Engl. 2016;55:3356–9.
Zhuang Y, Cao L. Sensitive fluorescence detection of etimicin based on derivatives of formaldehyde and acetylacetone. J Fluoresc. 2013;23:1–5.
Luo W, Li H, Zhang Y, Ang CY. Determination of formaldehyde in blood plasma by high-performance liquid chromatography with fluorescence detection. J Chromatogr B Biomed Sci Appl. 2001;753:253–7.
Yu D, Song L, Wang W, Guo C. Isolation and characterization of formaldehyde-degrading fungi and its formaldehyde metabolism. Environ Sci Pollut Res Int. 2014;21:6016–24.
Mei Y, Jiang C, Wan Y, Lv J, Jia J, Wang X, et al. Aging-associated formaldehyde-induced norepinephrine deficiency contributes to age-related memory decline. Aging Cell. 2015;14:659–68.
Qiang M, Xiao R, Su T, Wu BB, Tong ZQ, Liu Y, et al. A novel mechanism for endogenous formaldehyde elevation in SAMP8 mouse. J Alzheimers Dis. 2014;40:1039–53.
Teng S, Beard K, Pourahmad J, Moridani M, Easson E, Poon R, et al. The formaldehyde metabolic detoxification enzyme systems and molecular cytotoxic mechanism in isolated rat hepatocytes. Chem Biol Interact. 2001;130-132:285–96.
LaFerla FM, Green KN, Oddo S. Intracellular amyloid-beta in Alzheimer’s disease. Nat Rev Neurosci. 2007;8:499–509.
Murakami K, Irie K, Morimoto A, Ohigashi H, Shindo M, Nagao M, et al. Neurotoxicity and physicochemical properties of Abeta mutant peptides from cerebral amyloid angiopathy: implication for the pathogenesis of cerebral amyloid angiopathy and Alzheimer’s disease. J Biol Chem. 2003;278:46179–87.
Chen WT, Hong CJ, Lin YT, Chang WH, Huang HT, Liao JY, et al. Amyloid-beta (Abeta) D7H mutation increases oligomeric Abeta42 and alters properties of Abeta-zinc/copper assemblies. PLoS One. 2012;7:e35807.
Kaden D, Harmeier A, Weise C, Munter LM, Althoff V, Rost BR, et al. Novel APP/Abeta mutation K16N produces highly toxic heteromeric Abeta oligomers. EMBO Mol Med. 2012;4:647–59.
Bergman A, Religa D, Karlstrom H, Laudon H, Winblad B, Lannfelt L, et al. APP intracellular domain formation and unaltered signaling in the presence of familial Alzheimer’s disease mutations. Exp Cell Res. 2003;287:1–9.
Zhang Y, Dong Z, Song W. NLRP3 inflammasome as a novel therapeutic target for Alzheimer’s disease. Signal Transduct Target Ther. 2020;5:37.
Roberts AL, Johnson NJ, Cudkowicz ME, Eum KD, Weisskopf MG. Job-related formaldehyde exposure and ALS mortality in the USA. J Neurol Neurosurg Psychiatry. 2016;87:786–8.
Kilburn KH, Warshaw R, Thornton JC. Formaldehyde impairs memory, equilibrium, and dexterity in histology technicians: effects which persist for days after exposure. Arch Environ Health. 1987;42:117–20.
Kilburn KH. Neurobehavioral impairment and seizures from formaldehyde. Arch Environ Health. 1994;49:37–44.
Kilburn KH, Warshaw RH. Neurobehavioral effects of formaldehyde and solvents on histology technicians: repeated testing across time. Environ Res. 1992;58:134–46.
Tong Z, Wang W, Luo W, Lv J, Li H, Luo H, et al. Urine formaldehyde predicts cognitive impairment in post-stroke dementia and Alzheimer’s disease. J Alzheimers Dis. 2017;55:1031–8.
Perna RB, Bordini EJ, Deinzer-Lifrak M. A case of claimed persistent neuropsychological sequelae of chronic formaldehyde exposure: clinical, psychometric, and functional findings. Arch Clin Neuropsychol. 2001;16:33–44.
Tong Z, Zhang J, Luo W, Wang W, Li F, Li H, et al. Urine formaldehyde level is inversely correlated to mini mental state examination scores in senile dementia. Neurobiol Aging. 2011;32:31–41.
Tong Z, Han C, Luo W, Wang X, Li H, Luo H, et al. Accumulated hippocampal formaldehyde induces age-dependent memory decline. Age (Dordr). 2013;35:583–96.
Liu X, Zhang Y, Wu R, Ye M, Zhao Y, Kang J, et al. Acute formaldehyde exposure induced early Alzheimer-like changes in mouse brain. Toxicol Mech Methods. 2018;28:95–104.
Tan T, Zhang Y, Luo W, Lv J, Han C, Hamlin JNR, et al. Formaldehyde induces diabetes-associated cognitive impairments. FASEB J. 2018;32:3669–79.
Tong Z, Han C, Luo W, Li H, Luo H, Qiang M, et al. Aging-associated excess formaldehyde leads to spatial memory deficits. Sci Rep. 2013;3:1807.
Gubisne-Haberle D, Hill W, Kazachkov M, Richardson JS, Yu PH. Protein cross-linkage induced by formaldehyde derived from semicarbazide-sensitive amine oxidase-mediated deamination of methylamine. J Pharm Exp Ther. 2004;310:1125–32.
Wang F, Chen D, Wu P, Klein C, Jin C. Formaldehyde, epigenetics, and Alzheimer’s disease. Chem Res Toxicol. 2019;32:820–30.
Shindyapina AV, Komarova TV, Sheshukova EV, Ershova NM, Tashlitsky VN, Kurkin AV, et al. The antioxidant cofactor alpha-lipoic acid may control endogenous formaldehyde metabolism in mammals. Front Neurosci. 2017;11:651.
Ai L, Tan T, Tang Y, Yang J, Cui D, Wang R, et al. Endogenous formaldehyde is a memory-related molecule in mice and humans. Commun Biol. 2019;2:446.
Fiandaca MS, Kapogiannis D, Mapstone M, Boxer A, Eitan E, Schwartz JB, et al. Identification of preclinical Alzheimer’s disease by a profile of pathogenic proteins in neurally derived blood exosomes: a case-control study. Alzheimers Dement. 2015;11:600–607 e601.
Abd El-Aal SA, Abd El-Fattah MA, El-Abhar HS. CoQ10 augments rosuvastatin neuroprotective effect in a model of global ischemia via inhibition of NF-kappaB/JNK3/Bax and activation of Akt/FOXO3A/Bim cues. Front Pharm. 2017;8:735.
Momiyama Y. Serum coenzyme Q10 levels as a predictor of dementia in a Japanese general population. Atherosclerosis. 2014;237:433–4.
Dumont M, Kipiani K, Yu F, Wille E, Katz M, Calingasan NY, et al. Coenzyme Q10 decreases amyloid pathology and improves behavior in a transgenic mouse model of Alzheimer’s disease. J Alzheimers Dis. 2011;27:211–23.
Fei X, Yu Y, Di Y, Ai L, Yao D, Bai S, et al. A rapid and non-invasive fluorescence method for quantifying coenzyme Q10 in blood and urine in clinical analysis. J Clin Lab Anal. 2019;34:e23130.
Chang CT, Wu CS, Yang JT. Circular dichroic analysis of protein conformation: inclusion of the beta-turns. Anal Biochem. 1978;91:13–31.
Tjernberg LO, Callaway DJ, Tjernberg A, Hahne S, Lilliehook C, Terenius L, et al. A molecular model of Alzheimer amyloid beta-peptide fibril formation. J Biol Chem. 1999;274:12619–25.
Acknowledgements
We thank Prof. Lei Liu of Capital Medical University for his comments. This work was supported by the National Natural Science Foundation of China (82071214); the Beijing Natural Science Foundation of China (7172022); the Scientific Research Common Program of Beijing Municipal Commission of Education (KM201510025014); the Major Projects Fund of Beijing Institute for Brain Disorders (ZD2015-08); and the Canadian Institutes of Health Research (CIHR) Project Grant PJT-166127 (to WS). WS was the holder of the Tier 1 Canada Research Chair in Alzheimer’s Disease.
Author information
Authors and Affiliations
Contributions
WS and ZT conceived and designed the study and supervised all analyses. XF, YZ, YM, XY, WJ, LA, YY, HL, HL, WL, XY, JL and RH performed experiments. WS and ZT analyzed and contributed reagents /materials /analysis tools; XF, YZ, WS and ZT wrote the manuscript. All authors reviewed the manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Fei, X., Zhang, Y., Mei, Y. et al. Degradation of FA reduces Aβ neurotoxicity and Alzheimer-related phenotypes. Mol Psychiatry 26, 5578–5591 (2021). https://doi.org/10.1038/s41380-020-00929-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41380-020-00929-7
This article is cited by
-
Advancement in modulation of brain extracellular space and unlocking its potential for intervention of neurological diseases
Med-X (2024)
-
Advances in Molecular Psychiatry – March 2023: mitochondrial function, stress, neuroinflammation – bipolar disorder, psychosis, and Alzheimer’s disease
Molecular Psychiatry (2023)
-
Photobiomodulation for Alzheimer’s disease: photoelectric coupling effect on attenuating Aβ neurotoxicity
Lasers in Medical Science (2023)
-
Intermittent hypoxia treatment alleviates memory impairment in the 6-month-old APPswe/PS1dE9 mice and reduces amyloid beta accumulation and inflammation in the brain
Alzheimer's Research & Therapy (2021)
-
Molecular Psychiatry special issue: advances in Alzheimer’s disease
Molecular Psychiatry (2021)