Abstract
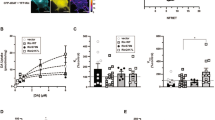
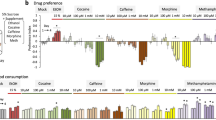
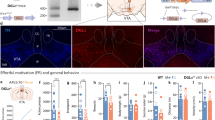
Reward modulates the saliency of a specific drug exposure and is essential for the transition to addiction. Numerous human PET–fMRI studies establish a link between midbrain dopamine (DA) release, DA transporter (DAT) availability, and reward responses. However, how and whether DAT function and regulation directly participate in reward processes remains elusive. Here, we developed a novel experimental paradigm in Drosophila melanogaster to study the mechanisms underlying the psychomotor and rewarding properties of amphetamine (AMPH). AMPH principally mediates its pharmacological and behavioral effects by increasing DA availability through the reversal of DAT function (DA efflux). We have previously shown that the phospholipid, phosphatidylinositol (4, 5)-bisphosphate (PIP2), directly interacts with the DAT N-terminus to support DA efflux in response to AMPH. In this study, we demonstrate that the interaction of PIP2 with the DAT N-terminus is critical for AMPH-induced DAT phosphorylation, a process required for DA efflux. We showed that PIP2 also interacts with intracellular loop 4 at R443. Further, we identified that R443 electrostatically regulates DA efflux as part of a coordinated interaction with the phosphorylated N-terminus. In Drosophila, we determined that a neutralizing substitution at R443 inhibited the psychomotor actions of AMPH. We associated this inhibition with a decrease in AMPH-induced DA efflux in isolated fly brains. Notably, we showed that the electrostatic interactions of R443 specifically regulate the rewarding properties of AMPH without affecting AMPH aversion. We present the first evidence linking PIP2, DAT, DA efflux, and phosphorylation processes with AMPH reward.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Winkelman TNA, Admon LK, Jennings L, Shippee ND, Richardson CR, Bart G. Evaluation of amphetamine-related hospitalizations and associated clinical outcomes and costs in the United States. JAMA Netw Open. 2018;1:e183758.
Berman S, O'Neill J, Fears S, Bartzokis G, London ED. Abuse of amphetamines and structural abnormalities in the brain. Ann N Y Acad Sci. 2008;1141:195–220.
UN Office DaC. World Drug Report 2017. Vienna, Austria: United Nations Publication; 2017. Report no.: 978-92-1-148291-1.
Freyberg Z, Sonders MS, Aguilar JI, Hiranita T, Karam CS, Flores J, et al. Mechanisms of amphetamine action illuminated through optical monitoring of dopamine synaptic vesicles in Drosophila brain. Nat Commun. 2016;7:10652.
Sulzer D, Sonders MS, Poulsen NW, Galli A. Mechanisms of neurotransmitter release by amphetamines: a review. Prog Neurobiol. 2005;75:406–33.
Hamilton PJ, Belovich AN, Khelashvili G, Saunders C, Erreger K, Javitch JA, et al. PIP2 regulates psychostimulant behaviors through its interaction with a membrane protein. Nat Chem Biol. 2014;10:582–9.
Pizzo AB, Karam CS, Zhang Y, Yano H, Freyberg RJ, Karam DS, et al. The membrane raft protein Flotillin-1 is essential in dopamine neurons for amphetamine-induced behavior in Drosophila. Mol Psychiatry. 2013;18:824–33.
Khoshbouei H, Sen N, Guptaroy B, Johnson L, Lund D, Gnegy ME, et al. N-terminal phosphorylation of the dopamine transporter is required for amphetamine-induced efflux. PLoS Biol. 2004;2:E78.
Cervinski MA, Foster JD, Vaughan RA. Psychoactive substrates stimulate dopamine transporter phosphorylation and down regulation by cocaine sensitive and protein kinase C dependent mechanisms. J Biol Chem. 2005;280:40442–9.
Fog JU, Khoshbouei H, Holy M, Owens WA, Vaegter CB, Sen N, et al. Calmodulin kinase II interacts with the dopamine transporter C terminus to regulate amphetamine-induced reverse transport. Neuron. 2006;51:417–29.
Johnson LA, Guptaroy B, Lund D, Shamban S, Gnegy ME. Regulation of amphetamine-stimulated dopamine efflux by protein kinase C beta. J Biol Chem. 2005;280:10914–9.
Binda F, Dipace C, Bowton E, Robertson SD, Lute BJ, Fog JU, et al. Syntaxin 1A interaction with the dopamine transporter promotes amphetamine-induced dopamine efflux. Mol Pharm. 2008;74:1101–8.
Cervinski MA, Foster JD, Vaughan RA. Syntaxin 1A regulates dopamine transporter activity, phosphorylation and surface expression. Neuroscience. 2010;170:408–16.
Mauna JC, Harris SS, Pino JA, Edwards CM, DeChellis-Marks MR, Bassi CD, et al. G protein betagamma subunits play a critical role in the actions of amphetamine. Transl Psychiatry. 2019;9:81.
Granas C, Ferrer J, Loland CJ, Javitch JA, Gether U. N-terminal truncation of the dopamine transporter abolishes phorbol ester- and substance P receptor-stimulated phosphorylation without impairing transporter internalization. J Biol Chem. 2003;278:4990–5000.
Foster JD, Pananusorn B, Vaughan RA. Dopamine transporters are phosphorylated on N-terminal serines in rat striatum. J Biol Chem. 2002;277:25178–86.
Cartier E, Hamilton PJ, Belovich AN, Shekar A, Campbell NG, Saunders C, et al. Rare autism-associated variants implicate syntaxin 1 (STX1 R26Q) phosphorylation and the dopamine transporter (hDAT R51W) in dopamine neurotransmission and behaviors. EBioMedicine. 2015;2:135–46.
Garcia-Olivares J, Baust T, Harris S, Hamilton P, Galli A, Amara SG, et al. Gbetagamma subunit activation promotes dopamine efflux through the dopamine transporter. Mol Psychiatry. 2017;22:1673–9.
Suh BC, Hille B. PIP2 is a necessary cofactor for ion channel function: how and why? Annu Rev Biophys. 2008;37:175–95.
McLaughlin S, Murray D. Plasma membrane phosphoinositide organization by protein electrostatics. Nature. 2005;438:605–11.
Kadamur G, Ross EM. Mammalian phospholipase C. Annu Rev Physiol. 2013;75:127–54.
Czech MP. PIP2 and PIP3: complex roles at the cell surface. Cell. 2000;100:603–6.
Ben-Aissa K, Patino-Lopez G, Belkina NV, Maniti O, Rosales T, Hao JJ, et al. Activation of moesin, a protein that links actin cytoskeleton to the plasma membrane, occurs by phosphatidylinositol 4,5-bisphosphate (PIP2) binding sequentially to two sites and releasing an autoinhibitory linker. J Biol Chem. 2012;287:16311–23.
Whorton MR, MacKinnon R. Crystal structure of the mammalian GIRK2 K+ channel and gating regulation by G proteins, PIP2, and sodium. Cell. 2011;147:199–208.
Thapa N, Anderson RA. PIP2 signaling, an integrator of cell polarity and vesicle trafficking in directionally migrating cells. Cell Adh Migr. 2012;6:409–12.
Buchmayer F, Schicker K, Steinkellner T, Geier P, Stubiger G, Hamilton PJ, et al. Amphetamine actions at the serotonin transporter rely on the availability of phosphatidylinositol-4,5-bisphosphate. PNAS. 2013;110:11642–7.
Khelashvili G, Stanley N, Sahai MA, Medina J, LeVine MV, Shi L, et al. Spontaneous inward opening of the dopamine transporter is triggered by PIP2-regulated dynamics of the N-terminus. ACS Chem Neurosci. 2015;6:1825–37.
Campbell NG, Shekar A, Aguilar JI, Peng D, Navratna V, Yang D, et al. Structural, functional, and behavioral insights of dopamine dysfunction revealed by a deletion in SLC6A3. PNAS. 2019;116:3853–62.
Kume K, Kume S, Park SK, Hirsh J, Jackson FR. Dopamine is a regulator of arousal in the fruit fly. J Neurosci. 2005;25:7377–84.
Friggi-Grelin F, Coulom H, Meller M, Gomez D, Hirsh J, Birman S. Targeted gene expression in Drosophila dopaminergic cells using regulatory sequences from tyrosine hydroxylase. J Neurobiol. 2003;54:618–27.
Wang JW, Beck ES, McCabe BD. A modular toolset for recombination transgenesis and neurogenetic analysis of Drosophila. PLoS One. 2012;7:e42102.
Hamilton PJ, Campbell NG, Sharma S, Erreger K, Herborg Hansen F, Saunders C, et al. De novo mutation in the dopamine transporter gene associates dopamine dysfunction with autism spectrum disorder. Mol Psychiatry. 2013;18:1315–23.
Cheng MH, Block E, Hu F, Cobanoglu MC, Sorkin A, Bahar I. Insights into the modulation of dopamine transporter function by amphetamine, orphenadrine, and cocaine binding. Front Neurol. 2015;6:134.
Wu EL, Cheng X, Jo S, Rui H, Song KC, Dávila‐Contreras EM, et al. CHARMM‐GUI membrane builder toward realistic biological membrane simulations. J Comput Chem. 2014;35:1997–2004.
Cheng MH, Ponzoni L, Sorkina T, Lee JY, Zhang S, Sorkin A, et al. Trimerization of dopamine transporter triggered by AIM-100 binding: Molecular mechanism and effect of mutations. Neuropharmacology. 2019;107676.
Phillips JC, Braun R, Wang W, Gumbart J, Tajkhorshid E, Villa E, et al. Scalable molecular dynamics with NAMD. J Comput Chem. 2005;26:1781–802.
Robbins J, Marsh SJ, Brown DA. Probing the regulation of M (Kv7) potassium channels in intact neurons with membrane-targeted peptides. J Neurosci. 2006;26:7950–61.
Yu K, Jiang T, Cui Y, Tajkhorshid E, Hartzell HC. A network of phosphatidylinositol 4,5-bisphosphate binding sites regulate gating of the Ca2+ activated Cl- channel ANO1 (TMEM16A). 2019; 625897. https://doi.org/10.1073/pnas.1904012116.
Yamamoto S, Seto ES. Dopamine dynamics and signaling in Drosophila: an overview of genes, drugs and behavioral paradigms. Exp Anim. 2014;63:107–19.
Claridge-Chang A, Roorda RD, Vrontou E, Sjulson L, Li H, Hirsh J, et al. Writing memories with light-addressable reinforcement circuitry. Cell. 2009;139:405–15.
Kirkhart C, Scott K. Gustatory learning and processing in the Drosophila mushroom bodies. J Neurosci. 2015;35:5950–8.
Kaun KR, Azanchi R, Maung Z, Hirsh J, Heberlein U. A Drosophila model for alcohol reward. Nat Neurosci. 2011;14:612–9.
Brand AH, Perrimon N. Targeted gene expression as a means of altering cell fates and generating dominant phenotypes. Development. 1993;118:401–15.
McClung C, Hirsh J. Stereotypic behavioral responses to free-base cocaine and the development of behavioral sensitization in Drosophila. Curr Biol. 1998;8:109–12.
Aguilar JI, Dunn M, Mingote S, Karam CS, Farino ZJ, Sonders MS, et al. Neuronal depolarization drives increased dopamine synaptic vesicle loading via VGLUT. Neuron. 2017;95:1074–88 e7.
Fleckenstein AE, Volz TJ, Riddle EL, Gibb JW, Hanson GR. New insights into the mechanism of action of amphetamines. Annu Rev Pharm Toxicol. 2007;47:681–98.
Arias-Carrion O, Stamelou M, Murillo-Rodriguez E, Menendez-Gonzalez M, Poppel E. Dopaminergic reward system: a short integrative review. Int Arch Med. 2010;3:24.
Scaplen KM, Kaun KR. Reward from bugs to bipeds: a comparative approach to understanding how reward circuits function. J Neurogenet. 2016;30:133–48.
Dubol M, Trichard C, Leroy C, Sandu AL, Rahim M, Granger B, et al. Dopamine transporter and reward anticipation in a dimensional perspective: a multimodal brain imaging study. Neuropsychopharmacology. 2018;43:820–7.
Schott BH, Minuzzi L, Krebs RM, Elmenhorst D, Lang M, Winz OH, et al. Mesolimbic functional magnetic resonance imaging activations during reward anticipation correlate with reward-related ventral striatal dopamine release. J Neurosci. 2008;28:14311–9.
Ja WW, Carvalho GB, Mak EM, de la Rosa NN, Fang AY, Liong JC, et al. Prandiology of Drosophila and the CAFE assay. PNAS. 2007;104:8253–6.
Diegelmann S, Jansen A, Jois S, Kastenholz K, Velo Escarcena L, Strudthoff N, et al. The CApillary FEeder assay measures food intake in Drosophila melanogaster. J Vis Exp. 2017;121:55024.
Devineni AV, Heberlein U. Preferential ethanol consumption in Drosophila models features of addiction. Curr Biol. 2009;19:2126–32.
Foltin RW. The behavioral pharmacology of anorexigenic drugs in nonhuman primates: 30 years of progress. Behav Pharm. 2012;23:461–77.
Cummings BS, Pati S, Sahin S, Scholpa NE, Monian P, Trinquero PM, et al. Differential effects of cocaine exposure on the abundance of phospholipid species in rat brain and blood. Drug Alcohol Depend. 2015;152:147–56.
Ross BM, Turenne SD. Chronic cocaine administration reduces phospholipase A(2) activity in rat brain striatum. Prostaglandins Leukot Ess Fat Acids. 2002;66:479–83.
Leussen DC, Psychostimulants Rong. Brain membrane lipids and dopamine transmission. J Biomolecular Res Therapeutics. 2016;5:1000143.
Marchant NJ, Kaganovsky K, Shaham Y, Bossert JM. Role of corticostriatal circuits in context-induced reinstatement of drug seeking. Brain Res. 2015;1628:219–32.
Ross BM, Moszczynska A, Peretti FJ, Adams V, Schmunk GA, Kalasinsky KS, et al. Decreased activity of brain phospholipid metabolic enzymes in human users of cocaine and methamphetamine. Drug Alcohol Depend. 2002;67:73–9.
Lee KH, Kim MY, Kim DH, Lee YS. Syntaxin 1A and receptor for activated C kinase interact with the N-terminal region of human dopamine transporter. Neurochem Res. 2004;29:1405–9.
Khelashvili G, Galli A, Weinstein H. Phosphatidylinositol 4,5-biphosphate (PIP(2)) lipids regulate the phosphorylation of syntaxin N-terminus by modulating both its position and local structure. Biochemistry. 2012;51:7685–98.
Amara SG, Kuhar MJ. Neurotransmitter transporters: recent progress. Annu Rev Neurosci. 1993;16:73–93.
Giros B, Caron MG. Molecular characterization of the dopamine transporter. Trends Pharm Sci. 1993;14:43–9.
Volkow ND, Fowler JS, Wang GJ. The addicted human brain viewed in the light of imaging studies: brain circuits and treatment strategies. Neuropharmacology. 2004;47 Suppl 1:3–13.
Cabib S, Orsini C, Le Moal M, Piazza PV. Abolition and reversal of strain differences in behavioral responses to drugs of abuse after a brief experience. Science. 2000;289:463–5.
O'Neill MF, Shaw G. Comparison of dopamine receptor antagonists on hyperlocomotion induced by cocaine, amphetamine, MK-801 and the dopamine D1 agonist C-APB in mice. Psychopharmacology. 1999;145:237–50.
Pignatelli M, Bonci A. Role of dopamine neurons in reward and aversion: a synaptic plasticity perspective. Neuron. 2015;86:1145–57.
Hu H. Reward and Aversion. Annu Rev Neurosci. 2016;39:297–324.
Acknowledgements
The authors would like to acknowledge Saunders Consulting for the help in editing this manuscript. Research reported in this publication was supported by NIH R01-DA038058 (AG), NIH R01-DA035263 (AG and HJGM), NIH K99/R00-DA045795 (PJH), NIH F31-MH114316 (JIA), NIH P41-GM103712 (IB), and NIH P30-DA035778 (IB). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Author information
Authors and Affiliations
Contributions
JIA, AG, and HJGM conceptualized the study. JIA, ANB, SJM, MHC, DZ, PJH, DJS, AS, and HJGM carried out the experiments. JIA and AG conducted formal analyses of the data. JIA, AG, and HJGM provided conceptual advice and edited the manuscript. JIA and AG prepared data and figures and wrote the manuscript. AG and HJGM acquired funding and supervised the study. All authors contributed to the editing and review of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Belovich, A.N., Aguilar, J.I., Mabry, S.J. et al. A network of phosphatidylinositol (4,5)-bisphosphate (PIP2) binding sites on the dopamine transporter regulates amphetamine behavior in Drosophila Melanogaster. Mol Psychiatry 26, 4417–4430 (2021). https://doi.org/10.1038/s41380-019-0620-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41380-019-0620-0
This article is cited by
-
The Role of the Dopamine Transporter in the Effects of Amphetamine on Sleep and Sleep Architecture in Drosophila
Neurochemical Research (2022)
-
Phosphatidylinositol 4,5-bisphosphate (PIP2) facilitates norepinephrine transporter dimerization and modulates substrate efflux
Communications Biology (2022)
-
Dopaminergic Ric GTPase activity impacts amphetamine sensitivity and sleep quality in a dopamine transporter-dependent manner in Drosophila melanogaster
Molecular Psychiatry (2021)
-
Identification by proximity labeling of novel lipidic and proteinaceous potential partners of the dopamine transporter
Cellular and Molecular Life Sciences (2021)