Abstract
Research suggests that endogenous opioids play a key role in the creation and maintenance of attachment bonds. Opioids acting at the μ-opioid receptor mediate reward and analgesia and are thus thought to underlie feelings of comfort and warmth experienced in the presence of close others. Disruption of μ-opioidergic activity increases separation distress in animals, suggesting that low opioid states may contribute to social pain. Accordingly, a functional μ-opioid receptor (OPRM1) polymorphism (C77G in primates, A118G in humans) affecting opioidergic signaling has been associated with separation distress and attachment behavior in nonhuman primates, and social pain sensitivity in humans. However, no research has examined the effects of this polymorphism on socioemotional experience, and specifically felt security, in daily interactions between romantic partners. Using an event-contingent recording method, members of 92 cohabiting romantic couples reported their felt security and quarrelsome behavior in daily interactions with each other for 20 days. Consistent with prior work, findings suggested that, relative to AA homozygotes, G allele carriers were more sensitive to their partners’ self-reported quarrelsome behaviors (e.g., criticism), showing a greater decline in felt security when their partners reported higher quarrelsome behavior than usual. This is the first study to link variation in OPRM1 with felt security toward romantic partners in everyday social interactions. More generally, this research supports the theory that the attachment system incorporated evolutionarily primitive pain-regulating opioidergic pathways. We also discuss implications of this work for understanding of differential vulnerability to health risks posed by social stress.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others
Notes
The original scale included four additional negatively worded items; however, these items have previously been found to have different psychometric properties (e.g., high skewness) and load on a separate factor from the positively worded items [29]. For these reasons, we chose to focus our analyses on the positively worded items, as per [29].
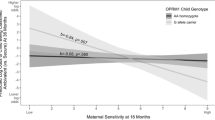
Figure 1 portrays results for women as actors and men as partners.
As noted, there was no gender difference in the moderation of the event-level association between a partner’s quarrelsome behavior and an actor’s felt security by actor genotype; however, men and women had different intercepts for felt security (as described in the subsequent text; also see Fig. S1).
References
Bowlby J. Attachment and loss: Vol. 1. Attachment. 2nd ed. New York, NY: Basic Books; 1969.
Eisenberger NI, Lieberman M, Williams K. Does rejection hurt? An fMRI study of social exclusion. Science. 2003;302:290–2.
MacDonald G, Leary MR. Why does social exclusion hurt? The relationship between social and physical pain. Psychol Bull. 2005;131:202–23.
Panksepp J. Affective neuroscience: the foundations of human and animal emotions. New York: Oxford University Press; 1998.
Tchalova K, Eisenberger NI. The shared neural substrates of physical and social pain. In: Williams KD, Nida SA, editors. Ostracism, exclusion, and rejection. New York: Routledge; 2017. p. 61–80.
Bowlby J. A secure base: clinical applications of attachment theory. London: Routledge; 1988.
Mikulincer M, Shaver PR. Attachment in adulthood: Structure, dynamics, and change. New York, NY: The Guilford Press; 2007.
Fields HL. Understanding how opioids contribute to reward and analgesia. Reg Anesth Pain Med. 2007;32:242–6.
Depue RA, Morrone-Strupinsky JV. A neurobehavioral model of affiliative bonding: Implications for conceptualizing a human trait of affiliation. Behav Brain Sci. 2005;28:313–50.
Herman BH, Panksepp J. Effects of morphine and naloxone on separation distress and approach attachment: Evidence for opiate mediation of social affect. Pharmacol Biochem Behav. 1978;9:213–20.
Matthews HWD Loss of morphine-induced analgesia, reward effect and withdrawal symptoms in mice lacking the mu-opioid receptor gene. Nature. 1996;383:819–23.
Panksepp J, Bean NJ, Bishop P, Vilberg T, Sahley TL. Opioid blockade and social comfort in chicks. Pharm Biochem Behav. 1980;13:673–83.
Kalin NH, Shelton SE, Barksdale CM, Lynn DE, Barksdale CM. Opiate modulation of separation-induced distress in non-human primates. Brain Res. 1988;440:285–92.
Panksepp J, Herman B, Conner R, Bishop P, Scott JP. The biology of social attachments: Opiates alleviate separation distress. Biol Psychiatry. 1978;13:607–18.
Inagaki TK, Ray LA, Irwin MR, Way BM, Eisenberger NI. Opioids and social bonding: Naltrexone reduces feelings of social connection. Soc Cogn Affect Neurosci. 2016;11:728–35.
Zubieta J-K, Ketter TA, Bueller JA, Xu Y, Kilbourn MR, Young EA, et al. Regulation of human affective responses by anterior cingulate and limbic mu-opioid neurotransmission. Arch Gen Psychiatry. 2003;60:1145–53.
Hsu DT, Sanford BJ, Meyers KK, Love TM, Hazlett KE, Wang H, et al. Response of the μ-opioid system to social rejection and acceptance. Mol Psychiatry. 2013;18:1211–7.
Bond C, LaForge KS, Tian M, Melia D, Zhang S, Borg L, et al. Single-nucleotide polymorphism in the human mu opioid receptor gene alters beta-endorphin binding and activity: possible implications for opiate addiction. Proc Natl Acad Sci USA. 1998;95:9608–13.
Zhang Y, Wang D, Johnson AD, Papp AC, Sadée W. Allelic expression imbalance of human mu opioid receptor (OPRM1) caused by variant A118G. J Biol Chem. 2005;280:32618–24.
Oertel BG, Kettner M, Scholich K, Renné C, Roskam B, Geisslinger G, et al. A common human micro-opioid receptor genetic variant diminishes the receptor signaling efficacy in brain regions processing the sensory information of pain. J Biol Chem. 2009;284:6530–5.
Sia AT, Lim Y, Lim ECP, Goh RWC, Law HY, Landau R, et al. A118G single nucleotide polymorphism of human mu-opioid receptor gene influences pain perception and patient-controlled intravenous morphine consumption after intrathecal morphine for postcesarean analgesia. Anesthesiology. 2008;109:520–6.
Menon S, Lea RA, Roy B, Hanna M, Wee S, Haupt LM, et al. The human μ-opioid receptor gene polymorphism (A118G) is associated with head pain severity in a clinical cohort of female migraine with aura patients. J Headache Pain. 2012;13:513–9.
Barr CS, Schwandt ML, Lindell SG, Higley JD, Maestripieri D, Goldman D, et al. Variation at the mu-opioid receptor gene (OPRM1) influences attachment behavior in infant primates. Proc Natl Acad Sci USA. 2008;105:5277–81.
Higham JP, Barr CS, Hoffman CL, Mandalaywala TM, Parker KJ, Maestripieri D. Mu-opioid receptor (OPRM1) variation, oxytocin levels and maternal attachment in free-ranging rhesus macaques Macaca mulatta. Behav Neurosci. 2011;125:131–6.
Boparai S, Borelli JL, Partington L, Smiley P, Jarvik E, Rasmussen HF, et al. Interaction between the opioid receptor OPRM1 gene and mother-child language style matching prospectively predicts children’s separation anxiety disorder symptoms. Res Dev Disabil. 2018;82:120–31.
Williams KD, Jarvis B. Cyberball: a program for use in research on interpersonal ostracism and acceptance. Behav Res Methods. 2006;38:174–80.
Way BM, Taylor SE, Eisenberger NI. Variation in the mu-opioid receptor gene (OPRM1) is associated with dispositional and neural sensitivity to social rejection. Proc Natl Acad Sci USA. 2009;106:15079–84.
Persson E, Asutay E, Heilig M, Löfberg A, Pedersen N, Västfjäll D, et al. Variation in the μ-opioid receptor gene (OPRM1) does not moderate social-rejection sensitivity in humans. Psychol Sci. 2019;30:1050–62.
Sadikaj G, Moskowitz DS, Zuroff DC. Felt security in daily interactions as a mediator of the effect of attachment on relationship satisfaction. Eur J Pers. 2015;29:187–200.
Kiecolt-Glaser JK, Malarkey WB, Cacioppo JT. Stressful personal relationships: immune and endocrine function. In: Handbook of human stress and immunity. Academic Press; San Diego, California, 1994. p. 321–39.
Gottman JM, Levenson RW. Assessing the role of emotion in marriage. Behav Assess. 1986;8:31–48.
Miller CW, Roloff ME. Gender and willingness to confront hurtful messages from romantic partners. Commun Q. 2005;53:323–37.
Vogel DL, Wester SR, Heesacker M, Madon S. Confirming gender stereotypes: a social role perspective. Sex Roles. 2003;48:519–28.
Zubieta J-K, Smith YR, Bueller JA, Xu Y, Kilbourn MR, Jewett DM, et al. Mu-opioid receptor-mediated antinociceptive responses differ in men and women. J Neurosci. 2002;22:5100–7.
Smith YR, Stohler CS, Nichols TE, Bueller JA, Koeppe RA, Zubieta J-K. Pronociceptive and antinociceptive effects of estradiol through endogenous opioid neurotransmission in women. J Neurosci. 2006;26:5777–85.
Olsen MB, Jacobsen LM, Schistad EI, Pedersen LM, Rygh LJ, Roe C, et al. Pain intensity the first year after lumbar disc herniation is associated with the A118G polymorphism in the opioid receptor mu 1 gene: evidence of a sex and genotype interaction. J Neurosci. 2012;32:9831–4.
Moskowitz DS, Sadikaj G. Event-contingent recording. In: Mehl MR, Conner TS, editors. Handbook of research methods for studying daily life. New York: The Guilford Press; 2012. p. 160–75.
Mathieu JE, Aguinis H, Culpepper SA, Chen G. Understanding and estimating the power to detect cross-level interaction effects in multilevel modeling. J Appl Psychol. 2012;97:951–66.
Spanier GB. Measuring dyadic adjustment: New scales for assessing the quality of marriage and similar dyads. J Marriage Fam. 1976;38:15.
Graffelman J. Exploring diallelic genetic markers: the Hardy Weinberg package. J Stat Softw. 2015;3:1–23.
Troisi A, Frazzetto G, Carola V, Di Lorenzo G, Coviello M, D’Amato FR, et al. Social hedonic capacity is associated with the A118G polymorphism of the mu-opioid receptor gene (OPRM1) in adult healthy volunteers and psychiatric patients. Soc Neurosci. 2011;6:88–97.
Moskowitz DS. Cross-situational generality and the interpersonal circumplex. J Pers Soc Psychol. 1994;66:921–33.
Carson RC. Interaction concepts of personality. Chicago: Aldine; 1969.
Kiesler DJ. The 1982 interpersonal circle: a taxonomy for complementarity in human transactions. Psychol Rev. 1983;90:185–214.
Horowitz LM, Rosenberg SE, Baer BA, Ureño G, Villaseñor VS. Inventory of interpersonal problems: psychometric properties and clinical applications. J Consult Clin Psychol. 1988;56:885–92.
Kenny DA, Kashy DA, Cook WL. Dyadic data analysis. New York, NY: The Guilford Press; 2006.
Laurenceau J-P, Bolger N. Analyzing diary and intensive longitudinal data from dyads. In: Mehl MR, Conner TS, editors. Handbook of research methods for studying daily life. New York, NY: Guilford Press; 2012. 407–22.
Muthén, LK, & Muthén BO Mplus User’s Guide. 6th ed. 2011 https://doi.org/10.1111/j.1600-0447.2011.01711.x.
Enders CK, Bandalos DL. The relative performance of full information maximum likelihood estimation for missing data in structural equation models. Struct Equ Model. 2001;8:430–57.
Satorra A, Bentler PM. Ensuring positiveness of the scaled difference chi-square test statistic. Psychometrika. 2010;75:243–8.
Arend MG, Schäfer T. Statistical power in two-level models: a tutorial based on Monte Carlo simulation. Psychol Methods. 2019;24:1–19.
Downey G, Feldman SI. Implications of rejection sensitivity for intimate relationships. J Pers Soc Psychol. 1996;70:1327–43.
Berenson KR, Gyurak A, Ayduk Ö, Downey G, Garner MJ, Mogg K, et al. Rejection sensitivity and disruption of attention by social threat cues. J Res Pers. 2009;43:1064.
Mikulincer M, Shaver P. Attachment theory and emotions in close relationships: Exploring the attachment-related dynamics of emotional reactions to relational events. Pers Relatsh. 2005;12:149–68.
Romero-Canyas R, Downey G, Berenson K, Ayduk O, Kang NJ. Rejection sensitivity and the rejection-hostility link in romantic relationships. J Pers. 2010;78:119–48.
Christensen A, Heavey CL. Gender and social structure in the demand/withdraw pattern of marital conflict. J Pers Soc Psychol. 1990;59:73–81.
Slavich GM, Tartter MA, Brennan PA, Hammen C. Endogenous opioid system influences depressive reactions to socially painful targeted rejection life events. Psychoneuroendocrinology. 2014;49:141–9.
Whitton SW, Rhoades GK, Whisman MA. Fluctuation in relationship quality over time and individual well-being. Personal Soc Psychol Bull. 2014;40:858–71.
Whitton SW, Whisman MA. Relationship satisfaction instability and depression. J Fam Psychol. 2010;24:791–4.
Heilig M, Epstein DH, Nader MA, Shaham Y. Time to connect: bringing social context into addiction neuroscience. Nat Rev Neurosci. 2016;17:1–22.
Linehan MM. Cognitive-behavioral treatment of borderline personality disorder. New York: Guilford Press; 1993. https://doi.org/10.1176/appi.psychotherapy.1994.48.1.155.
Acknowledgements
The authors would like to thank Kayleigh-Ann Clegg for her help with data collection.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Tchalova, K., Sadikaj, G., Moskowitz, D.S. et al. Variation in the μ-opioid receptor gene (OPRM1) and experiences of felt security in response to a romantic partner’s quarrelsome behavior. Mol Psychiatry 26, 3847–3857 (2021). https://doi.org/10.1038/s41380-019-0600-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41380-019-0600-4