Abstract
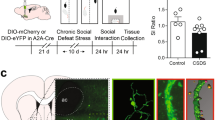
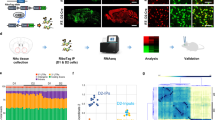
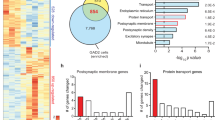
Depression alters the structure and function of brain reward circuitry. Preclinical evidence suggests that medium spiny neurons (MSNs) in the nucleus accumbens (NAc) undergo structural plasticity; however, the molecular mechanism and behavioral significance is poorly understood. Here we report that atrophy of D1, but not D2 receptor containing MSNs is strongly associated with social avoidance in mice subject to social defeat stress. D1-MSN atrophy is caused by cell-type specific upregulation of the GTPase RhoA and its effector Rho-kinase. Pharmacologic and genetic reduction of activated RhoA prevents depressive outcomes to stress by preventing loss of D1-MSN dendritic arbor. Pharmacologic and genetic promotion of activated RhoA enhances depressive outcomes by reducing D1-MSN dendritic arbor and is sufficient to promote depressive-like behaviors in the absence of stress. Chronic treatment with Rho-kinase inhibitor Y-27632 after chronic social defeat stress reverses depression-like behaviors by restoring D1-MSN dendritic complexity. Taken together, our data indicate functional roles for RhoA and Rho-kinase in mediating depression-like behaviors via dendritic remodeling of NAc D1-MSNs and may prove a useful target for new depression therapeutics.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Marcinkiewcz CA, Mazzone CM, D’Agostino G, Halladay LR, Hardaway JA, DiBerto JF et al. Serotonin engages an anxiety and fear-promoting circuit in the extended amygdala. Nature 2016;537:97–101.
Belzung C, Le Guisquet AM, Barreau S, Calatayud F. An investigation of the mechanisms responsible for acute fluoxetine-induced anxiogenic-like effects in mice. Behav Pharmacol. 2001;12:151–62.
Holtzheimer PE, Mayberg HS. Stuck in a rut: rethinking depression and its treatment. Trends Neurosci. 2011;34:1–9.
Zhu M-Y, Klimek V, Dilley GE, Haycock JW, Stockmeier C, Overholser JC et al. Elevated levels of tyrosine hydroxylase in the locus coeruleus in major depression. Biol Psychiatry 1999;46:1275–1286.
Liotti M, Mayberg HS. The role of functional neuroimaging in the neuropsychology of depression. J Clin Exp Neuropsychol. 2001;23:121–36.
Drevets WC. Neuroimaging and neuropathological studies of depression: implications for the cognitive-emotional features of mood disorders. Curr Opin Neurobiol. 2001;11:240–9.
Nestler EJ, Barrot M, DiLeone RJ, Eisch AJ, Gold SJ, Monteggia LM. Neurobiology of depression. Neuron 2002;34:13–25.
Floresco SB. The nucleus accumbens: an interface between cognition, emotion, and action. Annu Rev Psychol. 2015;66:25–52.
Russo SJ, Nestler EJ. The brain reward circuitry in mood disorders. Nat Rev Neurosci. 2013;14:609–25.
Lim BK, Huang KW, Grueter BA, Rothwell PE, Malenka RC. Anhedonia requires MC4R-mediated synaptic adaptations in nucleus accumbens. Nature. 2012;487:183–9.
Chaudhury D, Walsh JJ, Friedman AK, Juarez B, Ku SM, Koo JW et al. Rapid regulation of depression-related behaviours by control of midbrain dopamine neurons. Nature 2013;493:532–6.
Wilkinson MB, Dias C, Magida J, Mazei-Robison M, Lobo M, Kennedy P et al. A novel role of the WNT-Dishevelled-GSK3β signaling cascade in the mouse Nucleus Accumbens in a social defeat model of depression. J Neurosci 2011;31:9084–92.
Vialou V, Robison AJ, Laplant QC, Covington HE, Dietz DM, Ohnishi YN et al. DeltaFosB in brain reward circuits mediates resilience to stress and antidepressant responses. Nat Neurosci 2010;13:745–52.
Kim H-D, Hesterman J, Call T, Magazu S, Keeley E, Armenta K et al. SIRT1 Mediates Depression-Like Behaviors in the Nucleus Accumbens. J Neurosci 2016;36:8441–52.
Chandra R, Francis TC, Nam H, Riggs LM, Engeln M, Rudzinskas S et al. Reduced Slc6a15 in nucleus accumbens D2-neurons underlies stress susceptibility. J Neurosci 2017;3250–16.
Pizzagalli DA, Holmes AJ, Dillon DG, Goetz EL, Birk JL, Bogdan R et al. Reduced caudate and nucleus accumbens response to rewards in unmedicated individuals with major depressive disorder. Am J Psychiatry. 2009;166:702–10.
Smoski MJ, Felder J, Bizzell J, Green SR, Ernst M, Lynch TR et al. fMRI of alterations in reward selection, anticipation, and feedback in major depressive disorder. J Affect Disord 2009;118:69–78.
Stoy M, Schlagenhauf F, Sterzer P, Bermpohl F, H?gele C, Suchotzki K et al. Hyporeactivity of ventral striatum towards incentive stimuli in unmedicated depressed patients normalizes after treatment with escitalopram. J Psychopharmacol 2012;26:677–688.
Robinson OJ, Cools R, Carlisi CO, Sahakian BJ, Drevets WC. Ventral striatum response during reward and punishment reversal learning in unmedicated major depressive disorder. Am J Psychiatry. 2012;169:152–9.
Krishnan V, Han M-HH, Graham DL, Berton O, Renthal W, Russo SJ et al. Molecular adaptations underlying susceptibility and resistance to social defeat in brain reward regions. Cell 2007;131:391–404.
Misaki M, Suzuki H, Savitz J, Drevets WC, Bodurka J. Individual variations in nucleus accumbens responses associated with major depressive disorder symptoms. Sci Rep. 2016;6:21227.
Heshmati M, Aleyasin H, Menard C, Christoffel DJ, Flanigan ME, Pfau ML et al. Cell-type-specific role for nucleus accumbens neuroligin-2 in depression and stress susceptibility. Proc Natl Acad Sci USA 2018;115:1111–6.
Francis TC, Chandra R, Gaynor A, Konkalmatt P, Metzbower SR, Evans B et al. Molecular basis of dendritic atrophy and activity in stress susceptibility. Mol Psychiatry 2017;22:1512–19.
Muir J, Lorsch ZS, Ramakrishnan C, Deisseroth K, Nestler EJ, Calipari ES et al. In Vivo Fiber Photometry Reveals Signature of Future Stress Susceptibility in Nucleus Accumbens. Neuropsychopharmacology 2018;43:255–63.
Golden SA, Christoffel DJ, Heshmati M, Hodes GE, Magida J, Davis K et al. Epigenetic regulation of RAC1 induces synaptic remodeling in stress disorders and depression. Nat Med 2013;19:337–44.
Christoffel DJ, Golden SA, Heshmati M, Graham A, Birnbaum S, Neve RL et al. Effects of Inhibitor of κB Kinase Activity in the Nucleus Accumbens on Emotional Behavior. Neuropsychopharmacology 2012;37:2615–23.
Francis TC, Chandra R, Friend DM, Finkel E, Dayrit G, Miranda J et al. Nucleus accumbens medium spiny neuron subtypes mediate depression-related outcomes to social defeat stress. Biol Psychiatry 2015;77:212–22.
Wook Koo J, Labonté B, Engmann O, Calipari ES, Juarez B, Lorsch Z et al. Essential role of mesolimbic Brain-Derived Neurotrophic Factor in chronic social stress–induced depressive behaviors. Biol Psychiatry 2016;80:469–78.
Sun H, Damez-Werno DM, Scobie KN, Shao N-Y, Dias C, Rabkin J et al. ACF chromatin-remodeling complex mediates stress-induced depressive-like behavior. Nat Med 2015;21:1146–53.
Covington HE, Maze I, LaPlant QC, Vialou VF, Ohnishi YN, Berton O et al. Antidepressant actions of histone deacetylase inhibitors. J Neurosci 2009;29:11451–60.
Dias C, Feng J, Sun H, Shao NY, Mazei-Robison MS, Damez-Werno D et al. β-catenin mediates stress resilience through Dicer1/microRNA regulation. Nature 2014;516:51–5.
Gerfen CR, Surmeier DJ. Modulation of striatal projection systems by dopamine. Annu Rev Neurosci. 2011;34:441–66.
Kupchik YM, Brown RM, Heinsbroek JA, Lobo MK, Schwartz DJ, Kalivas PW. Coding the direct/indirect pathways by D1 and D2 receptors is not valid for accumbens projections. Nat Neurosci 2015;18:1230–32.
Villalba RM, Smith Y. Differential striatal spine pathology in Parkinson’s disease and cocaine addiction: a key role of dopamine? Neuroscience. 2013;251:2–20.
Gerfen CR. The neostriatal mosaic: multiple levels of compartmental organization in the basal ganglia. Annu Rev Neurosci. 1992;15:285–320.
Nicola SM. The nucleus accumbens as part of a basal ganglia action selection circuit. Psychopharmacology. 2007;191:521–50.
Maia TV, Frank MJ. From reinforcement learning models to psychiatric and neurological disorders. Nat Neurosci. 2011;14:154–62.
Fuchs E, Flugge G, Czeh B. Remodeling of neuronal networks by stress. Front Biosci. 2006;11:2746–58.
Magariños AM, McEwen BS. Stress-induced atrophy of apical dendrites of hippocampal CA3c neurons: comparison of stressors. Neuroscience. 1995;69:83–8.
Vyas A, Bernal S, Chattarji S. Effects of chronic stress on dendritic arborization in the central and extended amygdala. Brain Res. 2003;965:290–4.
Vyas A, Pillai AG, Chattarji S. Recovery after chronic stress fails to reverse amygdaloid neuronal hypertrophy and enhanced anxiety-like behavior. Neuroscience. 2004;128:667–73.
Muhammad A, Carroll C, Kolb B. Stress during development alters dendritic morphology in the nucleus accumbens and prefrontal cortex. Neuroscience. 2012;216:103–9.
Anacker C, Scholz J, O’Donnell KJ, Allemang-Grand R, Diorio J, Bagot RC et al. Neuroanatomic Differences Associated With Stress Susceptibility and Resilience. Biol Psychiatry 2016;79:840–849.
Christoffel DJ, Golden SA, Dumitriu D, Robison AJ, Janssen WG, Ahn HF et al. IκB kinase regulates social defeat stress-induced synaptic and behavioral plasticity. J Neurosci 2011;31:314–21.
Christoffel DJ, Golden SA, Russo SJ. Structural and synaptic plasticity in stress-related disorders. Rev Neurosci. 2011;22:535–49.
Kulkarni VA, Firestein BL. The dendritic tree and brain disorders. Mol Cell Neurosci. 2012;50:10–20.
Grieve SM, Korgaonkar MS, Koslow SH, Gordon E, Williams LM. Widespread reductions in gray matter volume in depression. NeuroImage Clin. 2013;3:332–9.
Koolschijn PCMP, van Haren NEM, Lensvelt-Mulders GJLM, Hulshoff Pol HE, Kahn RS. Brain volume abnormalities in major depressive disorder: a meta-analysis of magnetic resonance imaging studies. Hum Brain Mapp. 2009;30:3719–35.
Newey SE, Velamoor V, Govek E-E, Van Aelst L. Rho GTPases, dendritic structure, and mental retardation. J Neurobiol. 2005;64:58–74.
Nakayama AY, Harms MB, Luo L. Small GTPases Rac and Rho in the maintenance of dendritic spines and branches in hippocampal pyramidal neurons. J Neurosci. 2000;20:5329–38.
Peck JW, Oberst M, Bouker KB, Bowden E, Burbelo PD. The RhoA-binding protein, rhophilin-2, regulates actin cytoskeleton organization. J Biol Chem. 2002;277:43924–32.
Luo L. Actin cytoskeleton regulation in neuronal morphogenesis and structural plasticity. Annu Rev Cell Dev Biol. 2002;18:601–35.
Camera P, Schubert V, Pellegrino M, Berto G, Vercelli A, Muzzi P et al. The RhoA-associated protein Citron-N controls dendritic spine maintenance by interacting with spine-associated Golgi compartments. EMBO Rep 2008;9:384–92.
Chen H, Firestein BL. RhoA regulates dendrite branching in hippocampal neurons by decreasing cypin protein levels. J Neurosci. 2007;27:8378–86.
Sanz E, Yang L, Su T, Morris DR, McKnight GS, Amieux PS. Cell-type-specific isolation of ribosome-associated mRNA from complex tissues. Proc Natl Acad Sci 2009;106:13939–44.
Chandra R, Engeln M, Schiefer C, Patton MH, Martin JA, Werner CT et al. Drp1 Mitochondrial Fission in D1 Neurons Mediates Behavioral and Cellular Plasticity during Early Cocaine Abstinence. Neuron 2017;96:1327–1341.e6.
Golden SA, Covington HE, Berton O, Russo SJ. A standardized protocol for repeated social defeat stress in mice. Nat Protoc. 2011;6:1183–91.
Ferreira TA, Blackman A V, Oyrer J, Jayabal S, Chung AJ, Watt AJ et al. Neuronal morphometry directly from bitmap images. Nat Methods 2014;11:982–4.
Chandra R, Francis TC, Konkalmatt P, Amgalan A, Gancarz AM, Dietz DM et al. Opposing Role for Egr3 in Nucleus Accumbens Cell Subtypes in Cocaine Action. J Neurosci 2015;35:7927–37.
Chandra R, Lenz JD, Gancarz AM, Chaudhury D, Schroeder GL, Han M-H et al. Optogenetic inhibition of D1R containing nucleus accumbens neurons alters cocaine-mediated regulation of Tiam1. Front Mol Neurosci 2013;6:13.
Subauste MC, Von Herrath M, Benard V, Chamberlain CE, Chuang TH, Chu K et al. Rho family proteins modulate rapid apoptosis induced by cytotoxic T lymphocytes and Fas. J Biol Chem 2000;275:9725–33.
Hodes GE, Pfau ML, Purushothaman I, Ahn HF, Golden SA, Christoffel DJ et al. Sex Differences in Nucleus Accumbens Transcriptome Profiles Associated with Susceptibility versus Resilience to Subchronic Variable Stress. J Neurosci 2015;35:16362–76.
Gerfen CR, Paletzki R, Heintz N. GENSAT BAC Cre-recombinase driver lines to study the functional organization of cerebral cortical and basal ganglia circuits. Neuron. 2013;80:1368–83.
Magariños AM, McEwen BS, Flügge G, Fuchs E. Chronic psychosocial stress causes apical dendritic atrophy of hippocampal CA3 pyramidal neurons in subordinate tree shrews. J Neurosci. 1996;16:3534–40.
Chen Y, Dubé CM, Rice CJ, Baram TZ. Rapid loss of dendritic spines after stress involves derangement of spine dynamics by corticotropin-releasing hormone. J Neurosci. 2008;28:2903–11.
Chen Y, Kramár EA, Chen LY, Babayan AH, Andres AL, Gall CM et al. Impairment of synaptic plasticity by the stress mediator CRH involves selective destruction of thin dendritic spines via RhoA signaling. Mol Psychiatry 2013;18:485–96.
Swanson AM, DePoy LM, Gourley SL. Inhibiting Rho kinase promotes goal-directed decision making and blocks habitual responding for cocaine. Nat Commun. 2017;8:1861.
García-Rojo G, Fresno C, Vilches N, Díaz-Véliz G, Mora S, Aguayo F et al. The ROCK Inhibitor Fasudil Prevents Chronic Restraint Stress-Induced Depressive-Like Behaviors and Dendritic Spine Loss in Rat Hippocampus. Int J Neuropsychopharmacol 2017;20:336–45.
Xiao J, Zhu X, Wang Q, Zhang D, Cui C-S, Zhang P et al. Acute Effects of Rho-Kinase Inhibitor Fasudil on Pulmonary Arterial Hypertension in Patients With Congenital Heart Defects. Circ J 2015;79:1342–48.
Fukumoto Y, Yamada N, Matsubara H, Mizoguchi M, Uchino K, Yao A et al. Double-blind, placebo-controlled clinical trial with a rho-kinase inhibitor in pulmonary arterial hypertension. Circ J 2013;77:2619–25.
Acknowledgements
This work was funded by a One Mind/ Janssen Rising Star Translational Research Award (MKL), NIH R01MH106500 (MKL) and NIH F32MH116574 (MEF).
Author contributions
MEF and MKL designed the study. MEF, EJL, and HN conducted CSDS. MEF and RC prepared viral constructs. MEF and ME performed virus surgeries. MEF and MSM conducted morphology and immunolabeling experiments. MEF and EJL conducted ROCK activity and qPCR experiments. MEF conducted behavioral, pharmacologic, and RhoA activity experiments. MEF wrote the manuscript and prepared the figures with contributions from all authors. TCF conducted pilot experiments that inspired the study.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Electronic supplementary material
Rights and permissions
About this article
Cite this article
Fox, M.E., Chandra, R., Menken, M.S. et al. Dendritic remodeling of D1 neurons by RhoA/Rho-kinase mediates depression-like behavior. Mol Psychiatry 25, 1022–1034 (2020). https://doi.org/10.1038/s41380-018-0211-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41380-018-0211-5
This article is cited by
-
Social experience in adolescence shapes prefrontal cortex structure and function in adulthood
Molecular Psychiatry (2024)
-
G protein-biased LPAR1 agonism of prototypic antidepressants: Implication in the identification of novel therapeutic target for depression
Neuropsychopharmacology (2024)
-
Ventral striatal islands of Calleja neurons bidirectionally mediate depression-like behaviors in mice
Nature Communications (2023)
-
α-MSH-catabolic enzyme prolylcarboxypeptidase in nucleus accumbens shell ameliorates stress susceptibility in mice through regulating synaptic plasticity
Acta Pharmacologica Sinica (2023)
-
Plasticity of synapses and reward circuit function in the genesis and treatment of depression
Neuropsychopharmacology (2023)