Abstract
The advancing edge profile is a powerful determinant of tumor behavior in many organs. In this study, a grading system assessing the tumor-host interface was developed and tested in 181 pancreatic neuroendocrine tumors (PanNETs), 63 of which were <=2 cm. Three tumor slides representative of the spectrum (least, medium, and most) of invasiveness at the advancing edge of the tumor were selected, and then each slide was scored as follows. Well-demarcated/encapsulated, 1 point; Mildly irregular borders and/or minimal infiltration into adjacent tissue, 2 points; Infiltrative edges with several clusters beyond the main tumor but still relatively close, and/or satellite demarcated nodules, 3 points; No demarcation, several cellular clusters away from the tumor, 4 points; Exuberantly infiltrative pattern, scirrhous growth, dissecting the normal parenchymal elements, 5 points. The sum of the rankings on the three slides was obtained. Cases with scores of 3–6 were defined as “non/minimally infiltrative” (NI; n = 77), 7–9 as “moderately infiltrative” (MI; n = 68), and 10–15 as “highly infiltrative” (HI; n = 36). In addition to showing a statistically significant correlation with all the established signs of aggressiveness (grade, size, T-stage), this grading system was found to be the most significant predictor of adverse outcomes (metastasis, progression, and death) on multivariate analysis, more strongly than T-stage, while Ki-67 index did not stand the multivariate test. As importantly, cases <=2 cm were also stratified by this grading system rendering it applicable also to this group that is currently placed in “watchful waiting” protocols. In conclusion, the proposed grading system has a strong, independent prognostic value and therefore should be considered for integration into routine pathology practice after being evaluated in validation studies with larger series.
Similar content being viewed by others
Introduction
Tumor characteristics at the advancing edge of a neoplasm have been shown to be a very strong and reliable indicator of its biological behavior, including the propensity for dissemination. In endocrine organs such as the thyroid gland, where tumors are fairly common and thus are well studied for decades, the interaction of the tumor with the peripheral host tissues has been well-established1,2,3. In fact, this is not only one of the most reliable prognosticators, but actually also serves as the sole determinant in classifying a thyroid tumor as benign versus malignant in many cases; the diagnosis of follicular adenoma versus minimally invasive carcinoma versus widely invasive is largely based on the tumor-host interface4.
Advancing edge profile of neoplasms is being increasingly recognized and incorporated into management algorithms in other organs as well. For example, in gastrointestinal tract neoplasms, budding is one of the strongest prognosticators5,6,7,8,9,10. The findings of the invasion pattern vary by the organ. In the lungs, “STAS” (spread through air spaces) has been recently shown to be an important indicator of behavior11 and this is also now incorporated into routine reporting similar to other organs12. Even for pancreatic ductal adenocarcinomas, a classification system has been suggested by Japanese investigators based on the growth pattern13.
For pancreatic neuroendocrine tumors (PanNETs), infiltrating edge of the tumor was noted to be a potential indicator of behavior in some studies14. Recently, the presence of fibrous tissue within the tumor itself was also found to have a correlation with behavior15. However, the value of evaluation of the patterns of infiltration at the leading edge of a PanNET has not been studied systematically. One of the challenges has been the assessment of the degree of invasiveness when there is the heterogeneity of patterns in the tumor.
Prognostication of PanNETs and placement of patients into appropriate management protocols have been challenging. T-stage (mostly based on the tumor size) and grade (based on proliferative activity including Ki-67 and mitosis) have been regarded as the key prognostic factors; however, their value has been surprisingly limited16,17,18. Recently, for non-functional PanNETs that are smaller than 2 cm, a “watchful-waiting” approach has been proposed. However, this protocol was based on studies with limited follow-up, and there are major concerns regarding this approach because at least 10% of such cases prove to have metastasis if resected, and it is believed that many more will progress in follow-up. Unfortunately, there are currently no reliable factors to distinguish the cases that are more likely to progress, although recent evidence highlighted some molecular alterations such as DAXX mutations and ALT (“alternative lengthening of telomeres”) status associated with an increased risk of metastasis in small PanNETs19.
In this study, we devised a relatively simple scoring system in scaling the invasiveness at the advancing edge of PanNETs, incorporating tumoral heterogeneity in different sections, and investigated the clinicopathologic associations of this system with the established signs of aggressiveness as well as its potential value as an independent prognostic parameter.
Materials and methods
Case selection
This study was approved by the respective institutions’ review boards. Surgical pathology archives were searched for resected PanNETs. All diagnostic slides were culled and reviewed. Demographic and clinical information was obtained from pathology reports and institutions’ databases. Survival data were retrieved from National Death Notification System or by contacting the primary physicians of the patients.
Classification based on the infiltration pattern at the advancing edge
The principles of this proposed classification scheme were extrapolated from those used in colon20,21, a modified version of which has also been applied to the ampulla22. To minimize subjectivity and to maximally capture heterogeneity, a summative scoring system was developed as follows: Initially, three tumor slides representative of the spectrum (least, medium, and most) of peripheral infiltration were selected by eye-balling evaluation of the slides with tumor. Following that, the infiltration pattern was ranked microscopically from 1 to 5 (See diagram in Fig. 1; also detailed below) for each of the three selected slides of each case. For each selected slide, the highest infiltration pattern was considered, even if this infiltration was focal. The cases were evaluated by three of the authors (MDR, PB, and VA) who had also devised the scoring protocol in consensus evaluation of the cases. An interobserver agreement analysis was conducted separately to determine the reproducibility level (see the section below) in which two other authors also participated (OT and BS) in order to establish the usability of the criterion by various different observers.
1: Fully demarcated round and/or fully capsulated tumor. 2: Tumors with mildly irregular borders with early capsular penetration, in forms of clusters of cells minimally traversing beyond the imaginary line of the boundaries of the tumor but still mostly adjacent to or connected to the parent tumor. 3: The infiltration is observed either as large satellite nodules and/or irregular projections and small cluster spread into peri-tumoral regions but still relatively close and connected to the main lesion. 4: Infiltration into peri-tumoral region as small clusters/irregular units, but without extensive infiltration far away from tumor. 5: No demarcation; tumor with prominent infiltrative pattern, with many clusters away from main tumor and infiltration/dissection between normal elements or extensive infiltration into soft tissues.
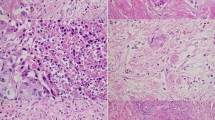
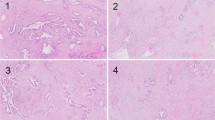
—1 point: Fully demarcated round and/or fully capsulated tumor (Figs. 2 and 3).
—2 points: Irregular borders with early capsular penetration in forms of clusters of cells minimally traversing beyond the imaginary line of the boundaries of the tumor but still mostly adjacent to or connected to the parent tumor (Fig. 4).
—3 points: Infiltrative edges, with the pattern of infiltration either as large satellite nodules and/or irregular projections and small clusters spread into peri-tumoral regions but still relatively close and connected to the main lesion (Fig. 5).
—4 points: Effacement of demarcation; infiltration into the peri-tumoral region as separate small clusters/irregular units, but without extensive infiltration far away from the tumor (Fig. 6).
—5 points: Total lack of demarcation; tumor with the prominent infiltrative pattern, with many clusters away from the main tumor and infiltration/dissection between normal acinar or isletic elements or extensive infiltration into soft tissues (Fig. 7).
The sum of the three rankings of each case (from 3 to 15) was defined as the case’s score. Cases with scores of 3–6 were defined as “non/minimally infiltrative (NI)”, 7–9 as “moderately infiltrative (MI)”, and 10–15 as “highly infiltrative (HI)”.
Testing of the interobserver agreement
This summative scoring system was devised to minimize the subjectivity of the interpretation such that it is practical and applicable by practicing pathologists. Nevertheless, in order to assess the interobserver agreement, 33 random cases were selected and re-evaluated by 3 of the observers and a reproducibility analysis was conducted by using Fleiss Kappa and Cronbach’s alpha coefficient.
Assessing the heterogeneity in the advancing edge
To obtain a general idea about the magnitude of heterogeneity of this parameter, the degree of variation in the grades between the three slides were analyzed arbitrarily and semi-quantitatively as follows:
A case was considered:
—Homogenous, if all three slides showed the same pattern (for example, 1 + 1 + 1). In other words, the difference between the slides was 0.
—Mildly heterogeneous, if the difference between the minimum and maximum scores was only one (for example, 3 + 3 + 4, 4 + 4 + 5).
—Moderately heterogeneous, if each slide showed a different invasiveness level (for example, 3 + 4 + 5), or there was at least 2 degree difference between any two slides (for example, 1 + 1 + 3).
—Markedly heterogeneous, if there was at least a 3 degree jump between scores (for example, 1 + 1 + 4 or 1 + 3 + 5).
Clinicopathologic parameters
The demographic information (age and gender) was extracted for each patient. The Ki-67 proliferation index and associated grade (G1–G3) were calculated using WHO-2019 criteria. The Ki-67 count was performed by using the manual quantification method previously described23. The size of the tumor was recorded from the macroscopic findings and verified by histologic examination. The pathologic stage was determined per the AJCC 8th edition, 2018. Follow-up information was obtained from patients’ charts or direct communication with their physicians.
Correlative analysis
Correlations were investigated between these groups and different clinical and pathologic parameters, (i.e., tumor size, T stage, clinical stage, lymph node metastasis, liver/distant metastasis, and Ki-67 proliferation index). Tumors with a size <=2 cm were additionally analyzed as a separate subset because currently “watchful-waiting” is being widely employed for these cases24.
Additionally, multivariate analysis was performed to test the independence of association of this proposed grading system with adverse behavior (lymph node metastasis, liver metastasis, recurrence, and death from disease) in comparison with namely grade and stage. Separately, a multivariate analysis was also performed taking only distant metastasis and disease-related death as the endpoints, while excluding lymph node metastasis. Of note, both metastases found at the time of resection as well as those that were discovered in follow-up were evaluated as adverse outcome events with the idea that the timing of their discovery is multifactorial (also dependent on the variably successful diagnostic modality approaches employed) and this timing may not necessarily reflect the biology of the disease but their mere occurrence does.
Statistical analysis
Descriptive statistics were presented to define continuous variables. The normality of continuous variables was investigated by Shapiro-Wilk’s test. The χ2 test was used for categorical variables along with Fisher Exact test, when applicable. Logistic regression was used to evaluate the effect of independent variables (which are found statistically significant at univariate analysis) on a dependent variable. Statistical significance was accepted when p-value was lower than 0.05. Statistical analysis was performed using the IBM Corp. Released 2010. IBM SPSS Statistics for Windows, Version 26.0. Armonk, NY: IBM Corp.
Results
Clinicopathologic data of the entire cohort
A total of 181 cases were analyzed. Except for two patients who presented with the clinical findings of insulinoma, none of the patients were known to have any specific syndrome or documented evidence of functionality. The female to male ratio was 1.2:1. The mean age of patients was 55 ± 14.9 years (range 17–84 years). The mean tumor size was 3.5 ± 2.3 cm (range: 0.5–11 cm). The mean Ki-67 index was 6.3 ± 11.8%. Based on the Ki-67 and mitotic index 54, 40, and 6% of the cases were graded as G1, G2, and G3. According to the current TNM classification, 33%, 28%, 38%, and 1% of tumors were staged as pT1, pT2, pT3, and pT4, respectively12.
At the time of resection, 31% of the cases had lymph node metastasis and 21% liver/distant metastasis. Among those with available clinical information (n = 155), at a median follow-up of 34 months, 13 patients died of disease-related causes.
Distribution of invasion patterns and their clinicopathologic associations
Applying the histopathologic infiltration grade system devised, 77 cases were qualified as non/minimally infiltrative (NI; score 3–6), 68 were moderately infiltrative (MI; score 7–9), and 36 were highly infiltrative (HI; score 10–15), respectively. See Fig. 8 for the gross representation of cases qualified for these categories.
Macroscopic reflections of respective categories: Non/minimal infiltrative examples showing round well demarcated tumors with smooth contours (A, B). Moderately infiltrative tumors characterized by irregularities at the borders showing infiltration beyond the boundaries of the main mass even though some maintaining a round architecture (C; infiltration at the upper right edge of the tumor), whereas some exhibiting more ill-defined appearance (D). Highly infiltrative tumors lacking demarcation and roundness, and instead showing a pattern characteristic of scirrhous-infiltrative neoplasms, mimicking pancreatic ductal adenocarcinoma (E, F).
At univariate analysis, there was a statistically significant direct correlation between the infiltration score and signs of aggressiveness in every parameter analyzed—except for mean tumor size—, each progressively increasing from NI, to MI, to HI. Table 1 reports detailed clinicopathologic features and comparative analysis.
Tumors <=2 cm
Sixty-three tumors were <=2 cm. Among those, 28 were qualified as NI (score 3–6), 27 as MI (score 7–9), and 8 as HI (Score 10–15). In 12 cases (19%; 1/28 from NI, 7/27 from MI, and 4/8 from HI), lymph node metastasis and/or distant/liver metastasis and/or death from the disease occurred.
At univariate analysis, there was a statistically significant direct correlation between the infiltration score and perineural, vascular invasion and the occurrence of adverse outcomes, each progressively increasing from NI, to MI, to HI, in this <=2 cm group as well. Table 2 reports detailed clinicopathologic features and comparative analysis.
Reproducibility of the scoring system
The kappa value for the interobserver agreement analysis was 0.526. Reliability between three observers was found at 0.913 level using Cronbach’s alpha coefficient, which is regarded as “excellent”.
Multivariate analysis
Multivariate analysis was performed to determine the correlation with adverse outcomes. The presence of lymph node metastasis, distant metastasis, and the occurrence of disease-related death was assigned as the endpoint of adverse outcomes. On multivariate logistic regression analysis, infiltration pattern was found to be the most significant predictor with HI group showing odds ratio of 16.4 compared to NI group (95% confidence interval 5.182–52.286; p < 0.001), and MI group showing odds ratio of 3.9 compared to NI group (95% confidence interval 1.577–10.002; p = 0.003). T-stage was also significant, with T3/4 cases having an odds ratio of 8.2 over T1 (95% confidence interval 2.908–23.582; p < 0.001), and T2 cases having an odds ratio of 3.1 over T1 (95% confidence interval 1.054–9.371; p = 0.04). Grade (based on Ki-67 and mitotic index), although significant at the univariate level (p < 0.001), did not stand up to the multivariate test (See Table 3 for results).
In the multivariate logistic regression analysis taking only distant metastasis and death of disease as the endpoints (excluding the lymph node metastasis), the infiltration pattern was still found to be a significant predictor with HI group showing an odds ratio of 3.4 compared to NI group (95% confidence interval 1.055–11.571; p = 0.04), and MI group showing odds ratio of 3.2 compared to NI group (95% confidence interval 1.154–9.389; p = 0.02). T-stage was also significant, with T3/4 cases having an odds ratio of 3.3 over T1/2 (95% confidence interval 1.394–7.841; p = 0.007). Grade (based on Ki-67 and mitotic index) did not stand up to the multivariate test in this analysis as well.
Heterogeneity/homogeneity
According to the arbitrary criteria described above, 84 (46%) cases were classified as homogenous (no variation between the slides), 71 (39%) as mildly heterogeneous, 20 (11%) as moderately heterogeneous, and 6 (4%) as markedly heterogeneous. The degree of heterogeneity/homogeneity in newly proposed groups is summarized in Table 4.
Discussion
This study elucidates that the degree of invasiveness at the advancing edge of the tumor is a strong reflection of biologic characteristics as well as the aggressiveness of PanNETs. The grading system developed to analyze the degree of invasiveness and tested in this study was found to be an independent prognosticator. We believe that this system should be strongly considered as an adjunct parameter in the evaluation of PanNETs, and should potentially be incorporated into the guidelines, once validated in other studies.
In the assessment of neoplasia, microscopic examination of the morphologic patterns and distribution of tumor characteristics has been an important part of pathology workup, and in fact, constituted the basis of histologic typing and grading, two of the most important tasks of pathologists in evaluating a tumor. For decades, this assessment was based on the overall characteristics of the tumor. Recently, however, it is being discovered that the infiltration patterns at the “advancing edge” of a given tumor provide precious hints concerning its behavior. Naturally, different tumor types exhibit different profiles in this regard such as “budding” in gastrointestinal tumors, “spread through air spaces” in pulmonary tumors, “microcystic, elongated and fragmented pattern” of invasion in uterine tumors, all of which have eventually become routine pathology reporting influencing management protocols in the past few years8,11,25. For the tumors that are prone to exhibit more expansive growth patterns similar to PanNETs, such as endocrine and gastrointestinal cancers, the degree of demarcation versus the infiltrative spread of the neoplastic cells at the periphery of the tumor has been found to yield invaluable prognostic information5,6,7,9. In fact, this is one of the main determinants of benign versus malignant diagnosis in thyroid neoplasms. Although there are exceptions, tumors with highly infiltrative patterns at the tumor-host interface tend to behave in an aggressive fashion, both locally and systemically compared to their more circumscribed counterparts.
Currently, the role pathology plays in the prognostication of PanNETs is mostly the assessment of the proliferative activity by counting Ki-67 and mitosis (for histologic grading) and TNM staging. Although the importance of infiltration patterns of PanNETs has been mentioned in the literature14, it has not been systematically analyzed and its associations with the clinical outcome have not been verified. The scoring system proposed in this study proved to correlate independently with known adverse survival determinants related to this tumor (see below).
First, this study elucidated that PanNETs from different patients indeed show substantial variation in the degree of invasiveness at the tumor-host interface. Using the scoring system developed, which is fairly simple and practical to employ, it was found that a substantial proportion of PanNETs (42%) are demarcated and are non/minimally infiltrative tumors, 38% have a moderate degree of invasiveness at their leading-edge although they form relatively compact lesions, and 20% exhibit a more scirrhous pattern and display a significant amount of invasion into the peripheral tissues. The respective macroscopic features of these categories also seem to reflect the infiltration patterns observed in microscopic examination (Fig. 8), which, in our opinion, should further be investigated in studies aiming at the radiologic-pathologic correlation of this phenomenon. If such correlations are strong, the pre-operative evaluation of PanNETs and management of the patients could change drastically.
This study also documents that there is a fair amount of intratumoral heterogeneity (variation in different zones of a given tumor) in the pattern of invasiveness at the tumor periphery. Therefore a grading system to assess the advancing edges of the tumor ought to have a scoring system that incorporates this intratumoral variability. Some degree of variation was present in different slides of a given tumor in more than 50% of the cases in this study. This heterogeneity was substantial in about 15% of the cases (with ≥2 degrees difference in between two slides of a case). The fact that the degree of intratumoral heterogeneity increases progressively from non/minimally infiltrative (with 34% of this group showing heterogeneity) to moderately infiltrative (66%), to the highly infiltrative (72%) is not surprising, and goes along with the progression scheme that at least some infiltrative PanNETs may be starting life as relatively more circumscribed lesions (displaying demarcated areas) but eventually developing more infiltrative areas. The univariate correlation of the invasiveness score with the size of the tumor also supports this impression. At the same time, its correlation with adverse outcomes independent of size also suggests that the invasiveness is not merely a result of enlargement of the tumor, but rather, that some tumors are inherently more infiltrative, even when they are small. The fact that the tumors <=2 cm could also be stratified in outcome by invasiveness score supports the concept that the invasiveness of the tumor is an innate tumor characteristic independent of the tumor size.
As documented in this study, the infiltration pattern in the advancing edge of PanNETs indeed has significant biologic/prognostic associations. The classification according to the proposed system (NI, MI, and HI groups) showed a stratification that correlated with every known histologic sign of aggressiveness in PanNETs, including tumor grade, T stage (size), Ki-67 index, metastasis, and progression. Moreover, in multivariate analysis, the “infiltration score” correlated independently with adverse outcomes (metastasis/progression), more successfully than the T stage, while grade did not even stand in the multivariate analysis. Accordingly, this study also emphasizes that it is important to sample PanNETs’ tumor-host interface well in the gross room, as is the standard for other endocrine tumors like thyroid or adrenal.
It would also be interesting to investigate the molecular, biological, and genetic associations of these infiltration patterns. For example, it has been documented that the rare serotonin-producing PanNETs are more prone to have sclerotic patterns26. Whether this intratumoral sclerosis also translates to more infiltrative patterns at the periphery, and whether they have any prognostic correlation (through or independently of the infiltration pattern) would be interesting to analyze. Similarly, recently potential theranostic markers have been identified in PanNETs, although some with conflicting results27,28,29,30. Nevertheless, these molecular alterations are likely to become a routine evaluation of PanNETs in the near future29 and it would be very interesting to appraise the association of these pathways with the infiltration patterns. Unfortunately, the hormonal status and molecular alterations were not specifically analyzed in the cases included in this study.
The findings of this study have the potential to make a major impact on the diagnosis and management of PanNETs. Currently, the prognostic factors in the evaluation of a primary PanNET, namely T-stage and grade have been rather inadequate. In fact, for practical purposes, G1 and G2 PanNETs are regarded as one category and managed very similarly31. Until recently, T-stage was also not a major determinant in management; however, a “watchful waiting” approach was recently proposed for PanNETs that measure <2 cm (T1) and is now widely employed throughout the world. However, strong concerns regarding this approach exist, considering that more than 10% of these cases prove to have lymph node metastasis when resected, and a higher number are expected to progress on longer follow-up. In this study, in nearly 20% of PanNETs <=2 cm, metastasis or death from the disease occurred. However, when broken down by the infiltration pattern, only 3.5% (n = 1/28) of the non/minimal invasion group had metastasis/progression. Whereas, in moderately infiltrative cases, this figure jumped to 26% (n = 7/27), and in the highly infiltrative group, half of the cases (n = 4/8; 50%) had metastasis/progression. Such differences were statistically significant. This is very important, because although attempts have been made to stratify this “<2 cm” category32 also with initial promising results19, widely accepted criteria were not put forward, and all non-functional PanNET patients with this tumor size are being offered observation. Based on the findings of this study, once the imaging correlates of these invasiveness patterns are established (which our preliminary observations prove to be highly promising in parallel with the gross findings; see Fig. 8), this criterion can be applied in the decision tree, where the non/minimally infiltrative candidates can be placed in less invasive treatment algorithms like watchful-waiting or radiofrequency/microwave ablation, while the more invasive ones, can be geared for surgery.
It is important to note that the proposed scoring system is fairly simple and easily adoptable to daily practice. The interobserver reliability was excellent per Cronbach’s alpha coefficient, and the kappa agreement level was 0.526, which is comparable to, and in fact better than for many of the grading systems widely used in daily practice in pathology, most of which have undergone extensive refinement over decades33,34,35,36,37,38,39,40,41. Assessment of tumor-host interface is something pathologists are very familiar with and have used for decades in various tumor types, especially in endocrine organs. This evaluation can be performed within minutes during routine examination of the slides: following a quick naked-eye evaluation of the circumscription of the tumor and selection of the highest, lowest, and medium slides, the tumor-host interface is examined microscopically at low power in each slide and scored accordingly. Based on the sum of the grades in the three slides, the case is then placed into one of three categories of “non/minimally infiltrative” (score 3–6), “moderately infiltrative” (7–9), and “highly infiltrative” (10–15). Especially when compared to the Ki-67 count, which failed to be an independent factor in the multivariate analysis, this invasiveness evaluation is much more practical. Performing accurate Ki-67 count requires a labor-intensive approach of the manual or automatic count since eye-balling is highly inaccurate and strongly discouraged unless the index is obviously in the extremes23. Therefore, this relatively simple morphology-based scoring system, applicable even in laboratories without access to Ki-67, may prove useful. Artificial intelligence algorithms may also be very helpful in assessing the invasiveness of PanNETs, and we have initiated a project to investigate this issue (study in progress).
In summary, the scoring system proposed in this study—which is based on the infiltration pattern of the advancing edges of PanNETs—was found to have significant prognostic correlations that are even stronger than currently used histologic grading and T staging systems. This system, if confirmed by validation studies, has the potential of being incorporated into daily practice and included in routine pathology reports. In the absence of one perfect prognosticator, this scoring system may be extremely useful in stratifying borderline patients into more appropriate management protocols.
Data availability
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Li, C., Xiang, J. & Wang, Y. Risk factors for predicting lymph nodes posterior to right recurrent laryngeal nerve (Ln-PRRLN) metastasis in thyroid papillary carcinoma: a meta-analysis. Int. J. Endocrinol. 2019, 7064328 (2019).
Jung, C. K. et al. Unique patterns of tumor growth related with the risk of lymph node metastasis in papillary thyroid carcinoma. Mod. Pathol. 23, 1201–1208 (2010).
Taşkın, O. et al. Tumor border pattern and size help predict lymph node status in papillary microcarcinoma: a clinicopathologic study. Ann. Diagn. Pathol. 48, 151592 (2020).
Lloyd, R., Osamura, R., Klöppel, G. & Rosai, J. WHO classification of tumours of the endocrine organs, 4th edn. (International Agency for Research on Cancer, 2017).
Gulluoglu, M. et al. Tumor budding is independently predictive for lymph node involvement in early gastric cancer. Int. J. Surg. Pathol. 23, 349–358 (2015).
Ohike, N. et al. Tumor budding as a strong prognostic indicator in invasive ampullary Adenocarcinomas. Am. J. Surg. Pathol. 34, 1417–1424 (2010).
Ito, T. et al. High tumor budding is a strong predictor of poor prognosis in the resected perihilar cholangiocarcinoma patients regardless of neoadjuvant therapy, showing survival similar to those without resection. BMC Cancer https://doi.org/10.1186/s12885-020-6695-9 (2020).
Lugli, A. et al. Recommendations for reporting tumor budding in colorectal cancer based on the International Tumor Budding Consensus Conference (ITBCC) 2016. Mod. Pathol. 30, 1299–1311 (2017).
Lawlor, R. T. et al. Prognostic role of high-grade tumor budding in pancreatic ductal adenocarcinoma: a systematic review and meta-analysis with a focus on epithelial to mesenchymal transition. Cancers (Basel) 11, 113 https://doi.org/10.3390/cancers11010113 (2019).
Losi, L. et al. Prognostic significance of histological features and biological parameters in stage I (pT1 and pT2) colorectal adenocarcinoma. Pathol. Res. Pract. 202, 663–670 (2006).
Shih, A. R. & Mino‐Kenudson, M. Updates on spread through air spaces (STAS) in lung cancer. Histopathology 77, 173–180 (2020).
Amin, M. B. et al. AJCC Cancer Staging Manual, 8th edn. (Springer International Publishing, American Joint Commission on Cancer, 2017).
Japan Pancreas Society. in Classification of Pancreatic Carcinoma, 4th English Edn., 70–101 (Kanehara & Co., Ltd, 2017).
Zhang, L. et al. KIT is an independent prognostic marker for pancreatic endocrine tumors: a finding derived from analysis of islet cell differentiation markers. Am. J. Surg. Pathol. 33, 1562–1569 (2009).
Chatterjee, D. et al. Intratumoral fibrosis and tumor growth pattern as prognostic factors in optimally resected pancreatic neuroendocrine neoplasms: an analysis of 168 cases. Pancreas 49, 255–260 (2020).
Shi, H., Zhang, Q., Han, C., Zhen, D. & Lin, R. Variability of the Ki-67 proliferation index in gastroenteropancreatic neuroendocrine neoplasms—a single-center retrospective study. BMC Endocr. Disord. 18, 51 https://doi.org/10.1186/s12902-018-0274-y (2018).
Nuñez‐Valdovinos, B. et al. Neuroendocrine tumor heterogeneity adds uncertainty to the World Health Organization 2010 Classification: real‐world data from the Spanish Tumor Registry (R‐GETNE). Oncologist 23, 422–432 (2018).
Liszka, Ł. Tissue heterogeneity contributes to suboptimal precision of WHO 2010 scoring criteria for Ki67 labeling index in a subset of neuroendocrine neoplasms of the pancreas. Pol. J. Pathol. 67, 318–331 (2016).
Pea, A. et al. Genetic analysis of small well-differentiated pancreatic neuroendocrine tumors identifies subgroups with differing risks of liver metastases. Ann. Surg. 271, 566–573 (2020).
Koelzer, V. H. & Lugli, A. The tumor border configuration of colorectal cancer as a histomorphological prognostic indicator. Front. Oncol. 4, 29 (2014).
Karamitopoulou, E. et al. Tumour border configuration in colorectal cancer: proposal for an alternative scoring system based on the percentage of infiltrating margin. Histopathology 67, 464–473 (2015).
Xue, Y. et al. Growth pattern of invasive ampullary carcinomas as demarcated versus infitlrative has significant prognostic correlation: a clinicopathologic analysis of 257 cases. United States & Canadian Academy of Pathology Annual Meeting Abstracts. Mod. Pathol. 31, 313 (2018).
Reid, M. D. et al. Calculation of the Ki67 index in pancreatic neuroendocrine tumors: a comparative analysis of four counting methodologies. Mod. Pathol. 28, 686–694 (2015).
Gaujoux, S. et al. Observational study of natural history of small sporadic nonfunctioning pancreatic neuroendocrine tumors. J. Clin. Endocrinol. Metab. 98, 4784–4789 (2013).
Pavlakis, K. et al. MELF invasion in endometrial cancer as a risk factor for lymph node metastasis. Histopathology 58, 966–973 (2011).
Mccall, C. M. et al. Serotonin expression in pancreatic neuroendocrine tumors correlates with a trabecular histologic pattern and large duct involvement. Hum. Pathol. 43, 1169–1176 (2012).
Chou, A. et al. ATRX loss is an independent predictor of poor survival in pancreatic neuroendocrine tumors. Hum. Pathol. 82, 249–257 (2018).
Roy, S. et al. Loss of chromatin-remodeling proteins and/or CDKN2A associates with metastasis of pancreatic neuroendocrine tumors and reduced patient survival times. Gastroenterology 154, 2060–2063.e8 (2018).
Hackeng, W. M. et al. Non-functional pancreatic neuroendocrine tumours: ATRX/DAXX and alternative lengthening of telomeres (ALT) are prognostically independent from ARX/PDX1 expression and tumour size. Gut https://doi.org/10.1136/gutjnl-2020-322595 (2021).
Jiao, Y. et al. DAXX/ATRX, MEN1 and mTOR pathway genes are frequently altered in pancreatic neuroendocrine tumors. Science 331, 1199–1203 (2011).
Ishida, H. & Lam, A. K. Y. Pancreatic neuroendocrine neoplasms: the latest surgical and medical treatment strategies based on the current World Health Organization classification. Crit. Rev. Oncol. Hematol. 145, 102835 https://doi.org/10.1016/j.critrevonc.2019.102835 (2020).
Lopez-Aguiar, A. G. et al. The conundrum of <2-cm pancreatic neuroendocrine tumors: a preoperative risk score to predict lymph node metastases and guide surgical management. Surgery 166, 15–21 (2019).
Melia, J. et al. A UK-based investigation of inter- and intra-observer reproducibility of Gleason grading of prostatic biopsies. Histopathology 48, 644–654 (2006).
Montironi, R. et al. Gleason grading of prostate cancer in needle biopsies or radical prostatectomy specimens: Contemporary approach, current clinical significance and sources of pathology discrepancies. BJU Int. 95, 1146–1152 (2005).
Rabe, K. et al. Interobserver variability in breast carcinoma grading results in prognostic stage differences. Hum. Pathol. 94, 51–57 (2019).
Meyer, J. S. et al. Breast carcinoma malignancy grading by Bloom-Richardson system vs proliferation index: Reproducibility of grade and advantages of proliferation index. Mod. Pathol. 18, 1067–1078 (2005).
Mahajan, D. et al. Reproducibility of the villous component and high-grade dysplasia in colorectal adenomas <1 cm: Implications for endoscopic surveillance. Am. J. Surg. Pathol. 37, 427–433 (2013).
Vennalaganti, P. et al. Discordance among pathologists in the United States and Europe in diagnosis of low-grade dysplasia for patients with Barrett’s esophagus. Gastroenterology 152, 564–570.e4 (2017).
Downs-Kelly, E. et al. Poor interobserver agreement in the distinction of high-grade dysplasia and adenocarcinoma in pretreatment Barrett’s esophagus biopsies. Am. J. Gastroenterol. 103, 2333–2340 (2008).
Kerkhof, M. et al. Grading of dysplasia in Barrett’s oesophagus: substantial interobserver variation between general and gastrointestinal pathologists. Histopathology 50, 920–927 (2007).
Dano, H. et al. Interobserver variability in upfront dichotomous histopathological assessment of ductal carcinoma in situ of the breast: the DCISion study. Mod. Pathol. 33, 354–366 (2020).
Acknowledgements
This study was presented in part at the 110th annual meeting of the United States and Canadian Academy of Pathology in March 2021.
Funding
The authors received no specific funding for this work.
Author information
Authors and Affiliations
Contributions
V. A., A. S., C. L., and O. B. conceptualized and designed the study; V. A., P. B., M. D. R., and C. S. devised the scoring protocol. M. D. R., P. B., B. P., Y. X., O. C. T., A. A., C. S., and V. A. identified the patients; D. D., A. B., E. B., A. B., B. M., C. B. L, B. P., and Y. X. compiled and organized the data; O. C. T., M. D. R., P. B., B. S., Y. X., Y. K., C. S., and V. A. reviewed histopathology. S.B. and A.B. performed statistical analyses. S. B., O. C. T, O. B., and V. A. analyzed data. O. C. T., M. D. R., B. P., O. B., and V. A. wrote the manuscript. D. D, E. B., B. P., and O. C. T. organized the figures and the tables. All the authors critically read, edited, and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval
This study was performed in accordance with the Declaration of Helsinki.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Taskin, O.C., Reid, M.D., Bagci, P. et al. Infiltration pattern predicts metastasis and progression better than the T-stage and grade in pancreatic neuroendocrine tumors: a proposal for a novel infiltration-based morphologic grading. Mod Pathol 35, 777–785 (2022). https://doi.org/10.1038/s41379-021-00995-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41379-021-00995-4
This article is cited by
-
Pancreatic Neuroendocrine Microtumors (WHO 2022) Are Not Always Low-Grade Neoplasms: A Case with a Highly Increased Proliferation Rate
Endocrine Pathology (2024)
-
Infiltrative Growth Predicts the Risk of Recurrence After Surgery in Well-Differentiated Non-Functioning Pancreatic Neuroendocrine Tumors
Endocrine Pathology (2023)
-
Silva cumulative score and its relationship with prognosis in Endocervical adenocarcinoma
BMC Cancer (2022)
-
“Pure” hepatoid tumors of the pancreas harboring CTNNB1 somatic mutations: a new entity among solid pseudopapillary neoplasms
Virchows Archiv (2022)