Abstract
The limited accuracy of endoscopic biopsy in detecting high-grade dysplasia or adenocarcinoma within ampullary adenoma or dysplasia has been reported. The natural history of ampullary dysplasia is also unclear, and there are no established guidelines to determine which patients with ampullary dysplasia require resection versus surveillance endoscopy. DNA flow cytometry was performed on 47 ampullary biopsies with low-grade dysplasia, 18 high-grade dysplasia, and 23 negative for dysplasia, as well as 11 cases of ampullary adenocarcinoma. Abnormal DNA content (aneuploidy or elevated 4N fraction > 6%) was identified in 9 (82%) of adenocarcinoma, 13 (72%) of high-grade dysplasia, 7 (15%) of low-grade dysplasia, and none (0%) of non-dysplastic mucosa. One-, 2-, and 7-year detection rates of high-grade dysplasia or adenocarcinoma in low-grade dysplasia patients with abnormal DNA content were 57%, 86%, and 88%, respectively, whereas low-grade dysplasia patients in the setting of normal DNA content had 1-, 2-, and 7-year detection rates of 10%, 10%, and 10%, respectively. The univariate and multivariate hazard ratios (HRs) for subsequent detection of high-grade dysplasia or adenocarcinoma in low-grade dysplasia patients with DNA content abnormality were 16.8 (p = <0.01) and 9.8 (p = <0.01), respectively. Among the 13 high-grade dysplasia patients with DNA content abnormality, 5 patients (38%) were subsequently found to have adenocarcinoma within a mean follow-up time of 3 months, whereas only 1 (20%) of the remaining 5 patients in the setting of normal DNA content developed adenocarcinoma in a month (HR = 2.6, p = 0.39). The overall 1- and 2-year detection rates of adenocarcinoma in all high-grade dysplasia patients (regardless of flow cytometric results) were 34% (95% confidence interval = 16–63%) and 47% (95% confidence interval = 23–79%), respectively. In conclusion, the majority of low-grade dysplasia patients (86%) in the setting of abnormal DNA content developed high-grade dysplasia or adenocarcinoma within 2 years and thus may benefit from resection, whereas those with normal DNA content may be followed with surveillance endoscopy. The presence of DNA content abnormality can also confirm a morphologic suspicion of high-grade dysplasia, which should be managed with resection, as nearly 50% of the high-grade dysplasia patients were found to have adenocarcinoma within 2 years.
Similar content being viewed by others
Introduction
Ampullary adenoma or dysplasia arises from the major duodenal papilla either sporadically or in the setting of familial adenomatous polyposis [1,2,3,4,5,6,7,8]. Since ampullary dysplasia can undergo malignant transformation to high-grade dysplasia or adenocarcinoma through the adenoma–carcinoma sequence, complete resection is generally advocated not only to prevent its progression to malignancy but also to exclude the possibility that the malignancy was missed on endoscopic biopsy [2, 9, 10]. The treatment options for ampullary dysplasia include surgical (pancreaticoduodenectomy [Whipple procedure] or transduodenal ampullectomy) and endoscopic resection. Pancreaticoduodenectomy has the advantage of low recurrence rate but has higher morbidity (25–65%) and mortality rates (1–10%) [2, 5, 11,12,13,14,15]. By contrast, local surgical (transduodenal ampullectomy) or endoscopic resection has lower morbidity (0–25%) and mortality rates (0–1%) at the expense of higher recurrence rates (5–33%), necessitating postoperative endoscopic surveillance for the detection of residual or recurrent neoplastic tissue [2, 5, 12, 13, 15,16,17,18,19,20,21,22]. Although the treatment has recently shifted toward minimally invasive endoscopic resection [10, 15, 18,19,20,21, 23,24,25], significant complications have been reported in up to 32% of patients, including acute pancreatitis, hemorrhage, perforation, cholangitis, and papillary stenosis [2, 15, 18,19,20,21, 23, 24]. As such, the issue today is not whether ampullary dysplasia can be resected, but rather which of the cases should be resected.
Guidelines have not been established to determine which patients with ampullary dysplasia require resection versus surveillance endoscopy. The time frame for malignant transformation from low-grade dysplasia to advanced neoplasia (high-grade dysplasia or adenocarcinoma) is also uncertain, and thus there is no consensus regarding the most appropriate surveillance interval. This is further complicated by the high false-negative rate (9–60%) of endoscopic biopsy specimens in detecting high-grade dysplasia or adenocarcinoma when compared with final resection specimens, underscoring the limited accuracy of endoscopic biopsy [2, 9, 10, 16, 18, 20, 21, 23, 25,26,27,28,29]. This is potentiated by significant histologic changes that can occur at the major papilla (due to marked inflammation, bile exposure, stones, or stents), which can limit accurate, reproducible histologic interpretation. Therefore, the overall interobserver agreement among pathologists in the histologic assessment of ampullary biopsies is only fair (kappa = 0.24) [30].
In this regard, additional biomarkers could assist in the histologic and risk assessment of these challenging cases. Various techniques, such as polymerase chain reaction analysis of K-ras mutations [31,32,33] and immunohistochemical staining (such as p53) [34,35,36,37], have been evaluated. Though these studies have demonstrated that such molecular abnormalities tend to occur at an early stage during the adenoma–carcinoma transition, patient outcome did not correlate with the K-ras or p53 mutation status. As an alternative, DNA flow cytometry can potentially be useful as an adjunct method for the diagnosis and/or risk assessment of ampullary dysplasia. Although we recently demonstrated its utility in dysplasia of Barrett’s esophagus [38], inflammatory bowel disease [39, 40], and stomach [41], reports of such analyses on ampullary dysplasia are currently not available. Thus, this study examines the potential value of DNA content analysis in the diagnosis and/or risk stratification of ampullary dysplasia using formalin-fixed paraffin-embedded tissue obtained from endoscopic biopsies.
Materials and methods
Patients and data collection
The pathology database (CoPath) at the University of California at San Francisco was searched over a 27-year period (between January 1990 and December 2017) to identify 65 ampullary biopsies with dysplasia, including 18 high-grade dysplasia from 18 patients and 47 low-grade dysplasia from 46 patients. As controls, 23 non-dysplastic ampullary biopsies (from 23 patients) and 11 adenocarcinoma cases (3 biopsies and 8 Whipple procedure specimens from 11 patients) were utilized. For inclusion in the study, patients must have had a biopsy diagnosis of dysplasia (either low- or high-grade dysplasia) with at least one follow-up biopsy or resection. Exclusion criteria included patients with a history of ampullary adenocarcinoma prior to the biopsy diagnosis of dysplasia or those who underwent curative treatments (either surgical or endoscopic resection) at the time of ampullary dysplasia diagnosis. All cases were re-reviewed by at least two gastrointestinal pathologists (WTC, GEK, and/or KWW), and the dysplastic cases were classified as either low- or high-grade dysplasia using published criteria [42]. Low-grade dysplasia was defined as having hyperchromatic, pencil-shaped nuclei limited to the basal half of cytoplasm without surface maturation or significant architectural alterations, whereas high-grade dysplasia was characterized by more severe cytologic (including enlarged, round nuclei and loss of polarity) and architectural changes (such as cribriform formation). The follow-up result of each biopsy was determined by reviewing all subsequent biopsies/resections for the occurrence of high-grade dysplasia or adenocarcinoma. When the same degree of dysplasia was identified in multiple biopsies from the same patient, the biopsy with the largest dysplastic area was selected for DNA flow cytometric analysis. Pertinent demographic (including age, gender, ethnicity, and history of familial adenomatous polyposis) and lesion-specific data (such as location, endoscopic appearance, and size) were collected by reviewing hospital electronic medical records. The location of each dysplastic lesion was classified into three types based on the combination of gross assessment and microscopic correlation if subsequent Whipple procedure specimens were available, or endoscopic/imaging findings for biopsy specimens without subsequent Whipple procedure specimens. These were designated as “ampulla” when the lesion was centered in the major papilla without obvious duodenal surface involvement, “peri-ampulla” when the lesion was predominantly on the duodenal surface surrounding the papilla, and “intra-ampulla” when there was extension of the lesion into the ampullary channel although the lesion was predominantly in the ampulla. The University of California at San Francisco Institutional Review Board for human subjects research approved our study (IRB # 16-21034).
DNA flow cytometry
As previously described [38, 39, 41], three to four 60-micron thick sections were cut from each tissue block, and a potential abnormal cell population was enriched by manually dissecting the areas of dysplasia from the background normal tissue. DAPI (4,6-diamidino-2-phenylindole; Accurate Chemical & Scientific Corporation, Westbury, NY) was used to stain each sample, which was subsequently analyzed with a BD LSRII S854 flow cytometer (BD Biosciences, San Jose, CA) using UV laser excitation. The computer program Multicycle (De Novo software, Glendale, CA) was used to analyze all DNA content histograms based on the published consensus guidelines for clinical DNA flow cytometry [43]. The ploidy status was defined as diploid when there was a single G0/G1 peak with a DNA index ranging from 1.9 to 2.1N [43]. Aneuploidy was characterized by the presence of one or more discrete G0/G1 peaks outside the diploid range (DNA index 1.9–2.1N), which were visually distinguishable from the normal diploid G0/G1 peak [43]. The presence of G2/tetraploid (4N, DNA index 1.9–2.1N) fraction >6% was also considered abnormal due to its proven efficacy in discriminating cancer risk in Barrett’s esophagus [44] and subsequent strong association with dysplasia or adenocarcinoma in the upper gastrointestinal tract [38, 41]. Two authors (WTC and PSR) interpreted all DNA histograms without knowledge of the histological diagnoses. Good-quality histograms were obtained from all 99 samples. Of note, based on the published consensus guidelines [43, 45], the minimum number of cells or nuclei to reproducibly detect DNA content abnormality is ~5000, which can be easily obtained from small mucosal biopsies. In fact, the majority (92%) of samples tested in this study (all but eight Whipple procedure specimens with adenocarcinoma) were from small mucosal biopsies, from which multiple thick sections were cut and macrodissected (without exhausting tissue in the majority of cases) to generate good-quality histograms, demonstrating the feasibility of this methodology. The number of cells or nuclei collected for DNA content measurements ranged from 5000 to 20,000 (not including debris and aggregates).
Statistical analysis
The primary statistical outcome was subsequent detection of high-grade dysplasia or adenocarcinoma from the date of initial dysplasia diagnosis. Since not all patients developed high-grade dysplasia or adenocarcinoma before follow-up ended, statistical analysis appropriate for censored data (Kaplan–Meier curves and Cox proportional hazards models) was utilized. Detection rates at specific time points were calculated from the Kaplan–Meier curves, and a null hypothesis of equal distribution of detection times was assessed using the log-rank test. Univariate and/or multivariate Cox proportional hazards models were used to determine the hazard ratios (HRs) for subsequent detection of high-grade dysplasia or adenocarcinoma among potential risk factors, including abnormal DNA content, age, gender, ethnicity, familial adenomatous polyposis, and endoscopic appearance of mass, as well as lesion location and size. The Asymptotic Wald test was used to calculate both 95% confidence intervals and p-values. P-values <0.05 were regarded as statistically significant. All analyses were performed using SAS 9.4.
Results
Clinicopathologic features of ampullary dysplasia
The clinicopathologic characteristics of our cohort are shown in Table 1. The patients comprised 41 males (42%) and 57 females (58%) with a mean age of 57 years (range: 9–88 years), but patients with high-grade dysplasia or adenocarcinoma (mean 66 and 67 years, respectively) were older than those with low-grade dysplasia (mean 50 years). Ampullary dysplasia was more common in Caucasian patients (62%) than non-Caucasian patients (38%). It usually presented as a polyp or mass (87%) centered in the major papilla without obvious duodenal surface involvement (84%) with a mean size of 2 cm (range: 0.2–5 cm), but it was also detected as a flat lesion or incidentally in normal-appearing mucosa (13%). Some cases were predominantly on the duodenal surface surrounding the papilla (peri-ampulla, 14%) or showed intraductal extension within the ampulla although the lesions were predominantly in the ampulla (intra-ampulla, 2%). Seventeen patients (17%) had a history of familial adenomatous polyposis. The mean follow-up time was 19 months (range: 2 days–255 months).
Normal DNA content in non-dysplastic ampullary biopsies
All DNA histograms from 23 non-dysplastic ampullary biopsies showed normal DNA content (Fig. 1; Table 2). None of these patients developed dysplasia or adenocarcinoma within a mean follow-up time of 10 months (range: 5 days–77 months).
DNA content abnormality in low-grade dysplasia
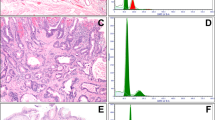
Seven (15%) of 47 low-grade dysplasia biopsies demonstrated DNA content abnormality (Fig. 2; Table 2). Aneuploidy was detected in four biopsies (Fig. 2b), whereas the remaining three biopsies demonstrated elevated 4N fraction (Fig. 2d). All seven patients (100%) were subsequently found to have high-grade dysplasia (five patients) or adenocarcinoma (two patients) within a mean follow-up time of 16 months (range: 5 days–85 months) (Table 2). They all underwent either pancreaticoduodenectomy (five patients, including two adenocarcinoma patients) or endoscopic resection (two patients). By contrast, only 3 (8%) of 39 low-grade dysplasia patients in the setting of normal DNA content developed high-grade dysplasia (two patients) or adenocarcinoma (one patient) within a mean follow-up time of 29 months (range: 2 days–255 months). They were managed with either pancreaticoduodenectomy (two patients, including one adenocarcinoma patient) or endoscopic resection (one patient). Among the remaining 36 low-grade dysplasia patients with normal DNA content, 15 patients underwent either pancreaticoduodenectomy (two patients) or endoscopic resection (13 patients). The other 21 patients were followed with surveillance endoscopy with a mean follow-up biopsies of 3 per patient (range: 1–8 follow-up biopsies per patient), and no high-grade dysplasia or adenocarcinoma was diagnosed within a mean follow-up time of 40 months (range: 2 days–213 months).
a, b Low-grade dysplasia is characterized by mild to moderate cytologic atypia with pseudostratified nuclei limited to the basal half of cytoplasm. There is a distinct lack of surface maturation without significant architectural disarray. A distinct aneuploid population (red) is present in this DNA histogram, in addition to a normal diploid population (green). c, d Another example of low-grade dysplasia. DNA histogram shows elevated 4N fraction >6%, but there is no distinct aneuploid peak
More interestingly, the presence of DNA content abnormality at baseline low-grade dysplasia was highly predictive of high-grade dysplasia or adenocarcinoma on follow-up with the estimated univariate and multivariate HRs of 16.8 (p = <0.01) and 9.8 (p = <0.01), respectively (Table 3). One-, 2-, and 7-year detection rates of high-grade dysplasia or adenocarcinoma in low-grade dysplasia patients with DNA content abnormality were 57%, 86%, and 88%, respectively, whereas 1-, 2-, and 7-year detection rates in low-grade dysplasia patients with normal DNA content were 10%, 10%, and 10%, respectively (Fig. 3b). The overall 1-, 2-, and 7-year detection rates of high-grade dysplasia or adenocarcinoma in all low-grade dysplasia patients (regardless of flow cytometric results) were 17%, 26%, and 38%, respectively (95% confidence intervals = [9–33%], [14–45%], and [19–68%], respectively) (Fig. 3a).
a Overall detection of high-grade dysplasia or adenocarcinoma in all low-grade dysplasia patients regardless of flow cytometric results. The detection rates of high-grade dysplasia or adenocarcinoma were 17%, 26%, and 38% within 1, 2, and 7 years, respectively. Each tick represents a patient being censored. b Detection of high-grade dysplasia or adenocarcinoma in low-grade dysplasia patients with abnormal DNA content at baseline. One-, 2-, and 7-year detection rates of high-grade dysplasia or adenocarcinoma in low-grade dysplasia patients with abnormal DNA content were 57%, 86%, and 88%, respectively, whereas 1-, 2-, and 7-year detection rates in low-grade dysplasia patients with normal DNA content were 10%, 10%, and 10%, respectively. Each tick represents a patient being censored
To identify other potential risk factors for subsequent detection of high-grade dysplasia or adenocarcinoma, hospital electronic medical records were reviewed to retrieve pertinent demographic (age, gender, ethnicity, and history of familial adenomatous polyposis) and lesion-specific data (location, endoscopic appearance, and size). In the univariate analysis, age (HR = 1.1, p = 0.01) and endoscopic appearance of mass (HR = 5.2, p = 0.04) were significantly associated with later development of high-grade dysplasia or adenocarcinoma (Table 3), but they did not achieve statistical significance in the multivariate analysis (p = 0.17 and 0.10, respectively). Similarly, gender (HR = 4.4, p = 0.16), ethnicity (HR = 2.2, p = 0.22), familial adenomatous polyposis (HR = 3.7, p = 0.10), lesion location (HR = 0.7, p = 0.72), and lesion size (HR = 1.6, p = 0.06) were not significant risk factors for subsequent detection of high-grade dysplasia or adenocarcinoma in the univariate Cox model. Of note, 11 (23%) of the 47 low-grade lesions were evaluated by endoscopic ultrasound, and there was no evidence of invasion in any of these cases.
DNA content abnormality in high-grade dysplasia
DNA content abnormality was detected in 13 (72%) of 18 high-grade dysplasia biopsies (Fig. 4; Table 2). Aneuploidy was detected in 11 biopsies, whereas the remaining two biopsies showed elevated 4N fraction. Five (38%) of the 13 high-grade dysplasia patients with DNA content abnormality were subsequently found to have adenocarcinoma within a mean follow-up time of 3 months (range: 10 days–15 months) (Table 2), whereas only one (20%) of the remaining five high-grade dysplasia patients in the setting of normal DNA content developed adenocarcinoma in a month. Overall, 6 (33%) of the 18 high-grade dysplasia patients developed adenocarcinoma within a mean follow-up time of 3 months (range: 10 days–15 months). These patients included two males and four females with a mean age of 68 years (range: 57–77 years), whereas the high-grade dysplasia patients without subsequent development of adenocarcinoma included seven males and five females with a mean age of 66 years (range: 47–84 years). Not surprisingly, five (83%) of the six patients with adenocarcinoma underwent pancreaticoduodenectomy. Of the remaining 12 high-grade dysplasia patients without subsequent detection of adenocarcinoma, 5 patients (42%) underwent endoscopic resection, but there was no evidence of resection in our database for the other 7 patients (58%) within a mean follow-up time of 5 months (range: 12 days–15 months).
a High-grade dysplasia shows severe cytologic atypia, including enlarged, round nuclei and loss of polarity. b DNA histogram shows a discrete aneuploid peak (red) that is distinct from a normal diploid population (green). c Overall detection of adenocarcinoma in all high-grade dysplasia patients regardless of flow cytometric results. One- and 2-year detection rates were 34% and 47%, respectively. Each tick represents a patient being censored. d Detection of adenocarcinoma in high-grade dysplasia patients with abnormal DNA content at baseline. One- and 2-year detection rates of adenocarcinoma in high-grade dysplasia patients with DNA content abnormality were 37% and 37%, respectively, whereas high-grade dysplasia patients in the setting of normal DNA content had 1- and 2-year detection rates of 25% and 25%, respectively
Based on the Kaplan–Meier curves, the overall detection rates of adenocarcinoma in all high-grade dysplasia patients (regardless of flow cytometric results) were 34% and 47% within 1 and 2 years, respectively (95% confidence intervals = [16–63%] and [23–79%], respectively) (Fig. 4c). More interestingly, 1- and 2-year detection rates of adenocarcinoma in high-grade dysplasia patients with DNA content abnormality were 37% and 37%, respectively, whereas high-grade dysplasia patients in the setting of normal DNA content had 1- and 2-year detection rates of 25% and 25%, respectively (Fig. 4d). However, this difference was not statistically significant (p = 0.39) based on the univariate Cox model, although abnormal DNA content was associated with an elevated HR of 2.6 (Table 4). Similarly, age (HR = 1.0, p = 0.61), gender (HR = 2.7, p = 0.28), ethnicity (HR = 0.8, p = 0.83), lesion location (HR = 0.9, p = 0.88), endoscopic appearance of mass (HR = 0.9, p = 0.91), and lesion size (HR = 0.7, p = 0.59) were not significantly associated with subsequent detection of adenocarcinoma. The HR and confidence interval for familial adenomatous polyposis were not estimable, because there was only a single patient with familial adenomatous polyposis in our high-grade dysplasia cohort.
DNA content abnormality in ampullary adenocarcinoma
Nine (82%) of 11 adenocarcinoma cases (three biopsies and eight Whipple procedure specimens) demonstrated DNA content abnormality (Fig. 5; Table 2). All nine cases showed aneuploidy.
Discussion
Although endoscopic biopsy is still the best method in obtaining accurate diagnostic information, it is known to have a high false-negative rate in detecting high-grade dysplasia or occult foci of adenocarcinoma in ampullary adenoma or dysplasia. This is a significant concern given the potential implications to patient care, and thus there is a need for additional biomarkers that can predict malignancy in ampullary dysplasia. In this regard, our data demonstrate that DNA content abnormality has the potential to serve such a purpose, as abnormal DNA content detected at baseline low-grade dysplasia was significantly associated with subsequent detection of high-grade dysplasia or adenocarcinoma with the estimated univariate and multivariate HRs of 16.8 (p = <0.01) and 9.8 (p = <0.01), respectively (Table 3). This is of particular importance, because controversy exists regarding the appropriate management of low-grade dysplasia (resection versus surveillance), even though pancreaticoduodenectomy has long been considered the standard procedure for high-grade dysplasia or adenocarcinoma. In fact, the majority of low-grade dysplasia patients (up to 86%) in the setting of abnormal DNA content were found to have high-grade dysplasia or adenocarcinoma within 2 years (Fig. 3b), and thus may benefit from endoscopic or surgical resection. Our results also suggest that given the low rate of progression for low-grade dysplasia patients with normal DNA content (up to 10% within 7 years), endoscopic surveillance (at least for the next few years) may be an acceptable alternative to resection. Although there is no established consensus regarding the most appropriate surveillance interval, it seems reasonable to perform a repeat endoscopy within 6 months (to look for high-grade dysplasia or adenocarcinoma) followed by follow-up examinations every 3–12 months for at least 2 years (depending on the degree of clinical suspicion), and then less frequently thereafter [2, 7, 15, 18, 19, 23]. Of note, we recently reported a similar association of DNA content abnormality and low-grade dysplasia with later development of high-grade dysplasia or cancer in Barrett’s esophagus [38], inflammatory bowel disease [39, 40], and stomach [41].
Furthermore, our results on low-grade dysplasia cases indicate that large-scale chromosomal alterations do occur in a subset of low-grade dysplasia cases (up to 15%), and that, given the rapid rate of progression of these cases to high-grade dysplasia or adenocarcinoma, such molecular events are occurring relatively late during the adenoma–carcinoma sequence, in spite of the appearance of low-grade dysplasia. This is further supported by the previous finding that allelic losses at chromosomes 17p and 18q are frequent alterations involved in the development of adenocarcinoma (53% and 34%, respectively) and significantly associated with shortened patient survival [32]. In that study, the relative risk of death within 5 years for adenocarcinoma patients with chromosome 17p loss was 11 times greater than that for those who retained both alleles (p = < 0.01). This is also consistent with the previous report that there is a significant difference in the median survival time between patients with diploid and aneuploid adenocarcinoma (159 versus 24 months, p = 0.03) [46].
As for high-grade dysplasia, although most clinicians would agree that patients with adenocarcinoma should be offered pancreaticoduodenectomy [47], some argue that endoscopic resection is a credible curative option for high-grade dysplasia [23, 48]. Since the diagnosis of high-grade dysplasia is an indication for at least endoscopic resection, it is critical that its diagnosis be confirmed prior to resection. In this regard, the majority of high-grade dysplasia biopsies (up to 72%) showed DNA content abnormality, while all of the non-dysplastic biopsies demonstrated normal DNA content, suggesting that DNA flow cytometry can provide confirmatory evidence to a morphologic suspicion of high-grade dysplasia in challenging cases, as well as in situations where there is discordant interpretation among gastrointestinal pathologists. In our series, we also note that up to 47% of high-grade dysplasia patients (regardless of flow cytometric results) were subsequently found to have adenocarcinoma within 2 years (Fig. 4c), arguing that these patients may benefit from aggressive surgical management (such as pancreaticoduodenectomy) rather than endoscopic resection. In support of this, Kim et al. demonstrated that up to 50% of high-grade dysplasia cases were found to have coexisting cancer [10]. Similarly, Meneghetti et al. [49] and Heidecke et al. [50] reported the rate of coexistence of adenocarcinoma in high-grade dysplasia patients as 100% and 44%, respectively. However, it should be noted that while DNA content abnormality in the setting of high-grade dysplasia was associated with an elevated HR of 2.6 for subsequent detection of adenocarcinoma, this finding did not achieve statistical significance in the present sample size (p = 0.39) (Table 4).
The high frequency of DNA content abnormality in high-grade dysplasia and ampullary adenocarcinoma in our series is consistent with the previous finding that adenocarcinoma with chromosomal instability represents the largest subtype of ampullary adenocarcinoma [32, 48,49,50,51,52]. In fact, the majority of adenocarcinoma cases (80–85%) are known to harbor chromosomal changes [32, 51,52,53,54,55], including early chromosome 5q loss as well as late allelic losses involving chromosomes 17p and 18q at the time of the adenoma–carcinoma transition. By contrast, a small subset of adenocarcinoma cases (15–20%) are known to exhibit microsatellite instability without significant chromosomal losses, and they are associated with a good prognosis [32, 52]. We also note that the frequency of DNA content abnormality in our adenocarcinoma cohort (82%) is higher than the previously reported (51%) [46]. This may be attributed to “dilutional effect” since this earlier study failed to macroscopically dissect malignant cells from the background normal cells, leading to an increase in the proportion of normal epithelial cells and thus reducing the sensitivity to detect malignant epithelial cells with abnormal DNA content.
It is interesting to note that although 17% of ampullary dysplasia in our cohort developed in the setting of familial adenomatous polyposis, there was no significant association between familial adenomatous polyposis and subsequent detection of high-grade dysplasia or adenocarcinoma (p = 0.10) (Table 3). However, this is consistent with the previous finding that the risk for high-grade dysplasia or adenocarcinoma is not significantly increased in patients with familial adenomatous polyposis [2, 5, 6, 8]. In fact, Burke et al. reported only 11% of patients with familial adenomatous polyposis (12 of 105 patients) had progression of histology when followed over 4 years [6]. Matsumoto et al. also demonstrated that of 12 low-grade dysplasia patients, only 3 patients (25%) developed high-grade dysplasia over 10 years [8]. Similarly, we found that none of the lesion-specific risk factors (such as location, endoscopic appearance, and size) was associated with subsequent detection of high-grade dysplasia or adenocarcinoma in the univariate and/or multivariate analysis. For instance, lesion size was not a significant risk factor in our cohort, but this may be due to the fact that the majority of lesions were relatively small with a mean size of 2 cm. In support of this, patients with tumor size ≤ 2.4 cm have been reported to have long-term successful outcomes after endoscopic resection [2, 5, 24]. Of note, although flat dysplasia in inflammatory bowel disease, especially in the setting of DNA content abnormality, has been shown to be significantly associated with subsequent detection of high-grade dysplasia or colorectal cancer [39], no such association was noted in ampullary dysplasia, as none of seven flat lesions (including five low- and two high-grade dysplasia) showed DNA content abnormality or developed high-grade dysplasia or adenocarcinoma on follow-up.
There are some limitations to our study. First, the University of California at San Francisco is a high-volume referral center, and hence referral bias cannot be ruled out, although the direction of such bias is difficult to predict in this setting. Second, we cannot entirely exclude the possibility that some of the demographic (age, gender, ethnicity, and history of familial adenomatous polyposis) and lesion-specific risk factors (location, endoscopic appearance, and size) may be associated with an increased risk for subsequent detection of high-grade dysplasia or adenocarcinoma with a longer follow-up time. Finally, some may argue that high-grade dysplasia/adenocarcinoma cases identified within the first year of ampullary dysplasia diagnosis may represent missed high-grade dysplasia/adenocarcinoma (rather than true neoplastic progression) at the time of biopsy. However, these types of cases are worthwhile to identify using DNA flow cytometry, given the limited accuracy of endoscopic biopsy in detecting high-grade dysplasia or adenocarcinoma within ampullary adenoma or dysplasia.
In conclusion, the presence of DNA content abnormality can identify a subset of low-grade dysplasia patients who are at increased risk for developing high-grade dysplasia or adenocarcinoma and thus may benefit from resection. By contrast, low-grade dysplasia patients in the setting of normal DNA content may be conservatively followed with surveillance endoscopy (or endoscopic resection in symptomatic patients), especially given the relatively high rates of complications following resection. The finding of DNA content abnormality can also provide confirmatory evidence to a morphologic suspicion of high-grade dysplasia, which should be managed with resection, as nearly 50% of the high-grade dysplasia patients were found to have adenocarcinoma within 2 years.
References
Offerhaus GJ, Giardiello FM, Krush AJ, Booker SV, Tersmette AC, Kelley NC, et al. The risk of upper gastrointestinal cancer in familial adenomatous polyposis. Gastroenterology. 1992;102:1980–2.
Chathadi KV, Khashab MA, Acosta RD, Chandrasekhara V, Eloubeidi MA, Faulx AL, et al. The role of endoscopy in ampullary and duodenal adenomas. Gastrointest Endosc. 2015;82:773–81.
Stolte M, Pscherer C. Adenoma-carcinoma sequence in the papilla of Vater. Scand J Gastroenterol. 1996;31:376–82.
van Stolk R, Sivak MVJ, Petrini JL, Petras R, Ferguson DR, Jagelman D. Endoscopic management of upper gastrointestinal polyps and periampullary lesions in familial adenomatous polyposis and Gardner’s syndrome. Endoscopy. 1987;19:19–22.
Martin JA, Haber GB. Ampullary adenoma: clinical manifestations, diagnosis, and treatment. Gastrointest Endosc Clin N Am. 2003;13:649–69.
Burke CA, Beck GJ, Church JM, van Stolk RU. The natural history of untreated duodenal and ampullary adenomas in patients with familial adenomatous polyposis followed in an endoscopic surveillance program. Gastrointest Endosc. 1999;49:358–64.
Hirota WK, Zuckerman MJ, Adler DG, Davila RE, Egan J, Leighton JA, et al. ASGE guideline: the role of endoscopy in the surveillance of premalignant conditions of the upper GI tract. Gastrointest Endosc. 2006;63:570–80.
Matsumoto T, Iida M, Nakamura S, Hizawa K, Yao T, Tsuneyoshi M, et al. Natural history of ampullary adenoma in familial adenomatous polyposis: reconfirmation of benign nature during extended surveillance. Am J Gastroenterol. 2000;95:1557–62.
Kim HN, Kim KM, Shin JU, Lee JK, Lee KT, Lee KH, et al. Prediction of carcinoma after resection in subjects with ampullary adenomas on endoscopic biopsy. J Clin Gastroenterol. 2013;47:346–51.
Kim JH, Kim JH, Han JH, Yoo BM, Kim MW, Kim WH. Is endoscopic papillectomy safe for ampullary adenomas with high-grade dysplasia? Ann Surg Oncol. 2009;16:2547–54.
Di Giorgio A, Alfieri S, Rotondi F, Prete F, Di Miceli D, Ridolfini MP, et al. Pancreatoduodenectomy for tumors of Vater’s ampulla: report on 94 consecutive patients. World J Surg. 2005;29:513–8.
Cahen DL, Fockens P, de Wit LT, Offerhaus GJ, Obertop H, Gouma DJ. Local resection or pancreaticoduodenectomy for villous adenoma of the ampulla of Vater diagnosed before operation. Br J Surg. 1997;84:948–51.
Tran TC, Vitale GC. Ampullary tumors: endoscopic versus operative management. Surg Innov. 2004;11:255–63.
Mendonça EQ, Bernardo WM, Moura EG, Chaves DM, Kondo A, Pu LZ, et al. Endoscopic versus surgical treatment of ampullary adenomas: a systematic review and meta-analysis. Clinics. 2016;71:28–35.
Chini P, Draganov PV. Diagnosis and management of ampullary adenoma: the expanding role of endoscopy. World J Gastrointest Endosc. 2011;3:241–7.
Posner S, Colletti L, Knol J, Mulholland M, Eckhauser F. Safety and long-term efficacy of transduodenal excision for tumors of the ampulla of Vater. Surgery. 2000;128:694–701.
Ceppa EP, Burbridge RA, Rialon KL, Omotosho PA, Emick D, Jowell PS, et al. Endoscopic versus surgical ampullectomy: an algorithm to treat disease of the ampulla of Vater. Ann Surg. 2013;257:315–22.
van der Wiel SE, Poley JW, Koch AD, Bruno MJ. Endoscopic resection of advanced ampullary adenomas: a single-center 14-year retrospective cohort study. Surg Endosc. 2018;33:1180–8.
Catalano MF, Linder JD, Chak A, Sivak MVJ, Raijman I, Geenen JE, et al. Endoscopic management of adenoma of the major duodenal papilla. Gastrointest Endosc. 2004;59:225–32.
Kang SH, Kim KH, Kim TN, Jung MK, Cho CM, Cho KB, et al. Therapeutic outcomes of endoscopic papillectomy for ampullary neoplasms: retrospective analysis of a multicenter study. BMC Gastroenterol. 2017;17:69.
Attila T, Parlak E, Alper E, Dişibeyaz S, Çiçek B, Ödemiş B. Endoscopic papillectomy of benign ampullary lesions: Outcomes from a multicenter study. Turk J Gastroenterol. 2018;29:325–34.
Hong S, Song KB, Lee YJ, Park KM, Kim SC, Hwang DW, et al. Transduodenal ampullectomy for ampullary tumors—single center experience of consecutive 26 patients. Ann Surg Treat Res. 2018;95:22–8.
Laleman W, Verreth A, Topal B, Aerts R, Komuta M, Roskams T, et al. Endoscopic resection of ampullary lesions: a single-center 8-year retrospective cohort study of 91 patients with long-term follow-up. Surg Endosc. 2013;27:3865–76.
Patel R, Davitte J, Varadarajulu S, Wilcox CM. Endoscopic resection of ampullary adenomas: complications and outcomes. Dig Dis Sci. 2011;56:3235–40.
Grobmyer SR, Stasik CN, Draganov P, Hemming AW, Dixon LR, Vogel SB, et al. Contemporary results with ampullectomy for 29 “benign” neoplasms of the ampulla. J Am Coll Surg. 2008;206:466–71.
Ryan DP, Schapiro RH, Warshaw AL. Villous tumors of the duodenum. Ann Surg. 1986;203:301–6.
Yamaguchi K, Enjoji M, Kitamura K. Endoscopic biopsy has limited accuracy in diagnosis of ampullary tumors. Gastrointest Endosc. 1990;36:588–92.
Elek G, Gyôri S, Tóth B, Pap A. Histological evaluation of preoperative biopsies from ampulla vateri. Pathol Oncol Res. 2003;9:32–41.
Lee SY, Jang KT, Lee KT, Lee JK, Choi SH, Heo JS, et al. Can endoscopic resection be applied for early stage ampulla of Vater cancer? Gastrointest Endosc. 2006;63:783–8.
Allard F, Goldsmith JD, Ayata G, Challies TL, Najarian RM, Nasser IA, et al. Intraobserver and interobserver variability in the assessment of dysplasia in ampullary mucosal biopsies. Am J Surg Pathol. 2018;42:1095–100.
Howe JR, Klimstra DS, Cordon-Cardo C, Paty PB, Park PY, Brennan MF. K-ras mutation in adenomas and carcinomas of the ampulla of vater. Clin Cancer Res. 1997;3:129–33.
Scarpa A, Di Pace C, Talamini G, Falconi M, Lemoine NR, Iacono C, et al. Cancer of the ampulla of Vater: chromosome 17p allelic loss is associated with poor prognosis. Gut. 2000;46:842–8.
Chung CH, Wilentz RE, Polak MM, Ramsoekh TB, Noorduyn LA, Gouma DJ, et al. Clinical significance of K-ras oncogene activation in ampullary neoplasms. J Clin Pathol. 1996;49:460–4.
Park SH, Kim YI, Park YH, Kim SW, Kim KW, Kim YT, et al. Clinicopathologic correlation of p53 protein overexpression in adenoma and carcinoma of the ampulla of Vater. World J Surg. 2000;24:54–9.
Sato T, Konishi K, Kimura H, Maeda K, Yabushita K, Tsuji M, et al. Adenoma and tiny carcinoma in adenoma of the papilla of Vater—p53 and PCNA. Hepatogastroenterology. 1999;46:1959–62.
Younes M, Riley S, Genta RM, Mosharaf M, Mody DR. p53 protein accumulation in tumors of the ampulla of Vater. Cancer. 1995;76:1150–4.
Takashima M, Ueki T, Nagai E, Yao T, Yamaguchi K, Tanaka M, et al. Carcinoma of the ampulla of Vater associated with or without adenoma: a clinicopathologic analysis of 198 cases with reference to p53 and Ki-67 immunohistochemical expressions. Mod Pathol. 2000;13:1300–7.
Choi WT, Tsai JH, Rabinovitch PS, Small T, Huang D, Mattis AN, et al. Diagnosis and risk stratification of Barrett’s dysplasia by flow cytometric DNA analysis of paraffin-embedded tissue. Gut. 2018;67:1229–38.
Tsai JH, Rabinovitch PS, Huang D, Small T, Mattis AN, Kakar S, et al. Association of aneuploidy and flat dysplasia with development of high-grade dysplasia or colorectal cancer in patients with inflammatory bowel disease. Gastroenterology. 2017;153:1492–5.
Wen KW, Rabinovitch PS, Wang D, Huang D, Mattis AN, Choi WT. Utility of DNA flow cytometric analysis of paraffin-embedded tissue in the risk stratification and management of ‘Indefinite for Dysplasia’ in patients with inflammatory bowel disease. J Crohns Colitis. 2019;13:472–81.
Wen KW, Rabinovitch PS, Huang D, Mattis AN, Lauwers GY, Choi WT. Use of DNA flow cytometry in the diagnosis, risk stratification, and management of gastric epithelial dysplasia. Mod Pathol. 2018;31:1578–87.
Schlemper RJ, Riddell RH, Kato Y, Borchard F, Cooper HS, Dawsey SM, et al. The Vienna classification of gastrointestinal epithelial neoplasia. Gut. 2000;47:251–5.
Shankey TV, Rabinovitch PS, Bagwell B, Bauer KD, Duque RE, Hedley DW, et al. Guidelines for implementation of clinical DNA cytometry. International Society for Analytical Cytology. Cytometry. 1993;14:472–7.
Rabinovitch PS, Longton G, Blount PL, Levine DS, Reid BJ. Predictors of progression in Barrett’s esophagus III: baseline flow cytometric variables. Am J Gastroenterol. 2001;96:3071–83.
Rabinovitch PS. Practical considerations for DNA content and cell cycle analysis. In: Bauer KD, Duque RE, Shankey TV, eds. Clinical flow cytometry: principles and applications. Baltimore: Williams and Wilkins; 1992. p. 117–42.
Shyr YM, Su CH, Wu LH, Li AF, Chiu JH, Lui WY. DNA ploidy as a major prognostic factor in resectable ampulla of Vater cancers. J Surg Oncol. 1993;53:220–5.
Seewald S, Omar S, Soehendra N. Endoscopic resection of tumors of the ampulla of Vater: how far up and how deep down can we go? Gastrointest Endosc. 2006;63:789–91.
Yoon SM, Kim MH, Kim MJ, Jang SJ, Lee TY, Kwon S, et al. Focal early stage cancer in ampullary adenoma: surgery or endoscopic papillectomy? Gastrointest Endosc. 2007;66:701–7.
Meneghetti AT, Safadi B, Stewart L, Way LW. Local resection of ampullary tumors. J Gastrointest Surg. 2005;9:1300–6.
Heidecke CD, Rosenberg R, Bauer M, Werner M, Weigert N, Ulm K, et al. Impact of grade of dysplasia in villous adenomas of Vater’s papilla. World J Surg. 2002;26:709–14.
Achille A, Baron A, Zamboni G, Di Pace C, Orlandini S, Scarpa A. Chromosome 5 allelic losses are early events in tumours of the papilla of Vater and occur at sites similar to those of gastric cancer. Br J Cancer. 1998;78:1653–60.
Achille A, Biasi MO, Zamboni G, Bogina G, Iacono C, Talamini G, et al. Cancers of the papilla of vater: mutator phenotype is associated with good prognosis. Clin Cancer Res. 1997;3:1841–7.
Achille A, Scupoli MT, Magalini AR, Zamboni G, Romanelli MG, Orlandini S, et al. APC gene mutations and allelic losses in sporadic ampullary tumours: evidence of genetic difference from tumours associated with familial adenomatous polyposis. Int J Cancer. 1996;68:305–12.
Scarpa A, Capelli P, Zamboni G, Oda T, Mukai K, Bonetti F, et al. Neoplasia of the ampulla of Vater. Ki-ras and p53 mutations. Am J Pathol. 1993;142:1163–72.
Scarpa A, Zamboni G, Achille A, Capelli P, Bogina G, Iacono C, et al. ras-family gene mutations in neoplasia of the ampulla of Vater. Int J Cancer. 1994;59:39–42.
Funding
This study was funded by the University of California at San Francisco Department of Pathology.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Wen, K.W., Kim, G.E., Rabinovitch, P.S. et al. Diagnosis, risk stratification, and management of ampullary dysplasia by DNA flow cytometric analysis of paraffin-embedded tissue. Mod Pathol 32, 1291–1302 (2019). https://doi.org/10.1038/s41379-019-0272-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41379-019-0272-2
This article is cited by
-
Adenomatous neoplasia in the papilla of Vater endoscopic and/or surgical resection?
Surgical Endoscopy (2022)
-
Utility of DNA flow cytometry in distinguishing between malignant and benign intrahepatic biliary lesions
Virchows Archiv (2020)