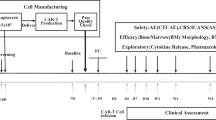
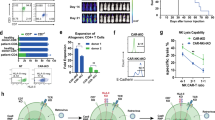
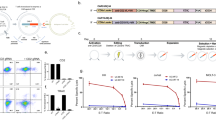
Abstract
T-cell acute lymphoblastic leukemia (T-ALL) represents an area of highly unmet medical needs. Once relapsed, patients have limited treatment options and poor prognosis. T-ALL antigens such as CD7 is extensively expressed in normal T cells and natural killer (NK) cells, and extending the success of CAR-T therapy to T cell malignancies was challenged by CAR-T cell fratricide, high production cost, and potential product contaminations. GC027 is an “off-the-shelf” allogeneic CD7 targeted CAR-T therapeutic product for T cell malignancies. It demonstrated superior cell expansion and antileukemia efficacy in mouse xenograft model. In our previous study, we observed promising efficacy results in the first two relapsed and refractory(R/R) T-ALL patients treated with GC027. In the expanded study, 11 out of 12 patients had rapid eradication of T-lymphoblasts and reached complete response within 1-month after GC027 infusion. GC027 cells expanded quickly beginning at infusion and reached to peak around 5–10 days after infusion. For most patients with a response(9/11), GC027 could not be detected via flow cytometry or qPCR 4 weeks after infusion. One patient had progression free survival of >3 years. With manageable toxicity profile, GC027 demonstrated superior clinical efficacy to standard chemotherapy regimens in (R/R) T cell malignancies.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
All data included in this study are available upon request by contact with the corresponding author.
References
Marks DI, Rowntree C. Management of adults with T-cell lymphoblastic leukemia. Blood. 2017;129:1134–42.
Marks DI, Paietta EM, Moorman AV, Richards SM, Buck G, DeWald G, et al. T-cell acute lymphoblastic leukemia in adults: clinical features, immunophenotype, cytogenetics, and outcome from the large randomized prospective trial (UKALL XII/ECOG 2993). Blood. 2009;114:5136–45.
Alcantara M, Tesio M, June CH, Houot R. CAR T-cells for T-cell malignancies: challenges in distinguishing between therapeutic, normal, and neoplastic T-cells. Leukemia. 2018;32:2307–15.
Li S, Wang X, Yuan Z, Liu L, Luo L, Li Y, et al. Eradication of T-ALL cells by CD7-targeted universal CAR-T cells and initial test of ruxolitinib-based CRS management. Clin Cancer Res. 2020;27:1242–46.
Rabinowich H, Pricop L, Herberman RB, Whiteside TL. Expression and function of CD7 molecule on human natural killer cells. J Immunol. 1994;152:517–26.
Gomes-Silva D, Srinivasan M, Sharma S, Lee CM, Wagner DL, Davis TH, et al. CD7-edited T cells expressing a CD7-specific CAR for the therapy of T-cell malignancies. Blood. 2017;130:285–96.
Freiwan A, Zoine JT, Crawford JC, Vaidya A, Schattgen SA, Myers JA, et al. Engineering naturally occurring CD7- T cells for the immunotherapy of hematological malignancies. Blood. 2022;140:2684–96.
Dai HP, Cui W, Cui QY, Zhu WJ, Meng HM, Zhu MQ, et al. Haploidentical CD7 CAR T-cells induced remission in a patient with TP53 mutated relapsed and refractory early T-cell precursor lymphoblastic leukemia/lymphoma. Biomark Res. 2022;10:6.
Lu Y, Liu Y, Wen S, Kuang N, Zhang X, Li J, et al. Naturally selected CD7 CAR-T therapy without genetic editing demonstrates significant antitumour efficacy against relapsed and refractory acute myeloid leukaemia (R/R-AML). J Transl Med. 2022;20:600.
Wong XFA, Ng J, Zheng S, Ismail R, Qian H, Campana D, et al. Development of an off-the-shelf chimeric antigen receptor (CAR)-T cell therapy for T-cell acute lymphoblastic leukemia (T-ALL) without gene editing. Blood. 2022;140:2358–9.
Watanabe N, Mo F, Zheng R, Ma R, Bray VC, van Leeuwen DG, et al. Feasibility and preclinical efficacy of CD7-unedited CD7 CAR T cells for T cell malignancies. Mol Ther. 2023;31:24–34.
Qasim W, Zhan H, Samarasinghe S, Adams S, Amrolia P, Stafford S, et al. Molecular remission of infant B-ALL after infusion of universal TALEN gene-edited CAR T cells. Sci Transl Med. 2017;9:eaaj2013.
Lee DW, Santomasso BD, Locke FL, Ghobadi A, Turtle CJ, Brudno JN, et al. ASTCT consensus grading for cytokine release syndrome and neurologic toxicity associated with immune effector cells. Biol Blood Marrow Transpl. 2019;25:625–38.
Wood BL. Principles of minimal residual disease detection for hematopoietic neoplasms by flow cytometry. Cytom B Clin Cytom. 2016;90(Jan):47–53.
Shum T, Omer B, Tashiro H, Kruse RL, Wagner DL, Parikh K, et al. Constitutive signaling from an engineered il7 receptor promotes durable tumor elimination by tumor-redirected T cells. Cancer Discov. 2017;7:1238–47.
Wang X, Li S, Gao L, Yuan Z, Wu K, Liu L, et al. Safety and efficacy results of GC027: The first-in-human, universal CAR-T cell therapy for adult relapsed/refractory T-cell acute lymphoblastic leukemia (r/r T-ALL). J Clin Oncol. 2020;38:3013.
Cooper ML, Choi J, Staser K, Ritchey JK, Devenport JM, Eckardt K, et al. An “off-the-shelf” fratricide-resistant CAR-T for the treatment of T cell hematologic malignancies. Leukemia. 2018;32:1970–83.
Pan J, Tan Y, Wang G, Deng B, Ling Z, Song W, et al. Donor-derived CD7 chimeric antigen receptor T cells for T-cell acute lymphoblastic leukemia: first-in-human, phase I trial. J Clin Oncol. 2021;39:3340–51.
Zhang M, Chen D, Fu X, Meng H, Nan F, Sun Z, et al. Autologous nanobody-derived fratricide-resistant CD7-CAR T-cell therapy for patients with relapsed and refractory T-cell acute lymphoblastic leukemia/lymphoma. Clin Cancer Res. 2022;28:2830–43.
Hu Y, Zhou Y, Zhang M, Zhao H, Wei G, Ge W, et al. Genetically modified CD7-targeting allogeneic CAR-T cell therapy with enhanced efficacy for relapsed/refractory CD7-positive hematological malignancies: a phase I clinical study. Cell Res. 2022;32:995–1007.
Zhang X, Zhou Y, Yang J, Li J, Qiu L, Ge W, et al. A novel universal CD7-Targeted CAR-T cell therapy for relapsed or refractory T-cell acute lymphoblastic leukemia and T-cell lymphoblastic lymphoma. Blood. 2022;140:4566–7.
Zhang X, Yang J, Li J, Qiu L, Li J, Lu P. Analysis of 53 patients with relapsed or refractory (R/R) T-cell acute lymphoblastic leukemia (T-ALL) and T-cell lymphoblastic lymphoma (T-LBL) treated with CD7-targeted CAR-T cell therapy. Blood. 2022;140:2369–70.
Acknowledgements
This study was supported by grants from the National Natural Science Foundation of China (No. 82100242), the Biomedical Major Project of Yunnan Province (No. 202102AA100011), the Basic Research Project of Yunnan Province(Youth program, No. 202101AU070090), the Young and Middle-aged Academic and Technical Leaders Reserve Talent Project of Yunnan Province (202205AC160074), the Application-review Project of 920th Hospital (No. 2020YGC01) and the Yunnan Applied Basic Research Projects-Union Foundation (No. 202201AY070001-280 and No. 202201AY070001-278).
Author information
Authors and Affiliations
Contributions
SW and XZ designed and leaded the clinical trial; SL and LLiu provided patient care and analyzed data. SL and XW wrote the manuscript. WC and XW designed the CAR-T product and in vitro experiments, and interpreted preclinical data. LS supervised the preclinical experiments and data interpretation. SL and Wenhui Huang conducted the preclinical experiments and data analysis. JH supervised CAR-T production. ZY, LLuo and YL provided clinical assistance. JL, GL, and ZL coordinated the study and data interpretation. WL reviewed the data and manuscript. LC, and PY and conducted clinical experiments. LG, JR, LZ, CL, XL, YW, DZ and YD provided clinical support and analyzed clinical data. LF and YC provided nursing support.
Corresponding authors
Ethics declarations
Competing interests
Gracell conceived and designed the GC027 product, performed the preclinical studies, manufactured the product and coordinated the study. The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Li, S., Wang, X., Liu, L. et al. CD7 targeted “off-the-shelf” CAR-T demonstrates robust in vivo expansion and high efficacy in the treatment of patients with relapsed and refractory T cell malignancies. Leukemia 37, 2176–2186 (2023). https://doi.org/10.1038/s41375-023-02018-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41375-023-02018-4