Abstract
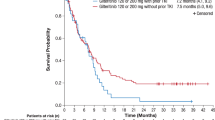
The real-world efficacy and safety of gilteritinib was assessed in an ambispective study that included 167 R/R FLT3-mutated AML patients. Among them, 140 received gilteritinib as single agent (cohort B), including 67 previously treated by intensive chemotherapy and midostaurin (cohort C). The main differences in patient characteristics in this study compared to the ADMIRAL trial were ECOG ≥ 2 (83.6% vs. 16.6%), FLT3-TKD mutation (21.0% vs. 8.5%), primary induction failure (15.0% vs. 40.0%) and line of treatment (beyond 2nd in 37.1% vs. 0.0%). The rates of composite complete remission, excluding those that occurred after hematopoietic stem cell transplantation (HSCT), were similar at respectively 25.4% and 27.5% in cohorts B and C. Median overall survival (OS) for these two groups was also similar at respectively 6.4 and 7.8 months. Multivariate analyses for prognostic factors associated with OS identified female gender (HR 1.61), adverse cytogenetic risk (HR 2.52), and allogenic HSCT after gilteritinib (HR 0.13). Although these patients were more heavily pretreated, these real-world data reproduce the results of ADMIRAL and provide new insights into the course of patients previously treated by intensive chemotherapy and midostaurin and beyond the 2nd line of treatment who can benefit from treatment in an outpatient setting.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
Data availability
Some de-identified data will be shared with other researchers upon reasonable request to the corresponding authors (pierre-yves.dumas@u-bordeaux.fr). The sharing will require a detailed proposal to the study investigators, and a data transfer agreement must be signed.
References
Bullinger L, Döhner K, Döhner H. Genomics of acute myeloid leukemia diagnosis and pathways. J Clin Oncol J Am Soc Clin Oncol. 2017;35:934–46.
Schlenk RF, Döhner K, Krauter J, Fröhling S, Corbacioglu A, Bullinger L, et al. Mutations and treatment outcome in cytogenetically normal acute myeloid leukemia. N. Engl J Med. 2008;358:1909–18.
Smith CC, Wang Q, Chin C-S, Salerno S, Damon LE, Levis MJ, et al. Validation of ITD mutations in FLT3 as a therapeutic target in human acute myeloid leukaemia. Nature. 2012;485:260–3.
Cocciardi S, Dolnik A, Kapp-Schwoerer S, Rücker FG, Lux S, Blätte TJ, et al. Clonal evolution patterns in acute myeloid leukemia with NPM1 mutation. Nat Commun. 2019;10:2031.
Schmalbrock LK, Dolnik A, Cocciardi S, Sträng E, Theis F, Jahn N, et al. Clonal evolution of acute myeloid leukemia with FLT3-ITD mutation under treatment with midostaurin. Blood. 2021;137:3093–104.
Schnittger S, Schoch C, Dugas M, Kern W, Staib P, Wuchter C, et al. Analysis of FLT3 length mutations in 1003 patients with acute myeloid leukemia: correlation to cytogenetics, FAB subtype, and prognosis in the AMLCG study and usefulness as a marker for the detection of minimal residual disease. Blood. 2002;100:59–66.
Döhner H, Estey E, Grimwade D, Amadori S, Appelbaum FR, Büchner T, et al. Diagnosis and management of AML in adults: 2017 ELN recommendations from an international expert panel. Blood. 2017;129:424–47.
Perl AE, Martinelli G, Cortes JE, Neubauer A, Berman E, Paolini S, et al. Gilteritinib or chemotherapy for relapsed or refractory FLT3-Mutated AML. N. Engl J Med. 2019;381:1728–40.
Stone RM, Mandrekar SJ, Sanford BL, Laumann K, Geyer S, Bloomfield CD et al. Midostaurin plus chemotherapy for acute myeloid leukemia with a FLT3 Mutation. N Engl J Med 2017. https://doi.org/10.1056/NEJMoa1614359.
Arber DA, Orazi A, Hasserjian R, Thiele J, Borowitz MJ, Le Beau MM, et al. The 2016 revision to the World Health Organization classification of myeloid neoplasms and acute leukemia. Blood. 2016;127:2391–405.
Grimwade D, Walker H, Oliver F, Wheatley K, Harrison C, Harrison G, et al. The importance of diagnostic cytogenetics on outcome in AML: analysis of 1,612 patients entered into the MRC AML 10 trial. The Medical Research Council Adult and Children’s Leukaemia Working Parties. Blood. 1998;92:2322–33.
Numan Y, Abdel Rahman Z, Grenet J, Boisclair S, Bewersdorf JP, Collins C, et al. Gilteritinib clinical activity in relapsed/refractory FLT3 mutated acute myeloid leukemia previously treated with FLT3 inhibitors. Am J Hematol. 2022;97:322–8.
Perl AE, Altman JK, Cortes J, Smith C, Litzow M, Baer MR, et al. Selective inhibition of FLT3 by gilteritinib in relapsed or refractory acute myeloid leukaemia: a multicentre, first-in-human, open-label, phase 1-2 study. Lancet Oncol. 2017. https://doi.org/10.1016/S1470-2045(17)30416-3.
Perl AE, Hosono N, Montesinos P, Podoltsev N, Martinelli G, Panoskaltsis N, et al. Clinical outcomes in patients with relapsed/refractory FLT3-mutated acute myeloid leukemia treated with gilteritinib who received prior midostaurin or sorafenib. Blood Cancer J. 2022;12:84.
Wiernik PH, Sun Z, Cripe LD, Rowe JM, Fernandez HF, Luger SM, et al. Prognostic effect of gender on outcome of treatment for adults with acute myeloid leukaemia. Br J Haematol. 2021;194:309–18.
Dumas P-Y, Naudin C, Martin-Lannerée S, Izac B, Casetti L, Mansier O, et al. Hematopoietic niche drives FLT3-ITD acute myeloid leukemia resistance to quizartinib via STAT5- and hypoxia- dependent up-regulation of AXL. Haematologica 2019. https://doi.org/10.3324/haematol.2018.205385.
Dumas P-Y, Villacreces A, Guitart AV, Ali EH, Massara L, Mansier O, et al. Dual inhibition of FLT3 and AXL by gilteritinib overcomes hematopoietic niche-driven resistance mechanisms in FLT3-ITD acute myeloid leukemia. Clin Cancer Res Off J Am Assoc Cancer Res. 2021; clincanres.3114.2020.
McMahon CM, Ferng T, Canaani J, Wang ES, Morrissette JJD, Eastburn DJ, et al. Clonal selection with RAS pathway activation mediates secondary clinical resistance to selective FLT3 inhibition in acute myeloid leukemia. Cancer Discov. 2019;9:1050–63.
Smith CC, Levis MJ, Perl AE, Hill JE, Rosales M, Bahceci E. Molecular profile of FLT3-mutated relapsed/refractory patients with AML in the phase 3 ADMIRAL study of gilteritinib. Blood Adv. 2022;6:2144–55.
Joshi SK, Nechiporuk T, Bottomly D, Piehowski PD, Reisz JA, Pittsenbarger J, et al. The AML microenvironment catalyzes a stepwise evolution to gilteritinib resistance. Cancer Cell. 2021;39:999–1014.e8.
Pratz KW, Cherry M, Altman JK, Cooper BW, Cruz JC, Jurcic JG, et al. A Phase 1 Study of Gilteritinib in combination with induction and consolidation chemotherapy in patients with newly diagnosed AML: Final results. Blood. 2020;136:16–17.
Wang ES, Montesinos P, Minden MD, Lee J-H, Heuser M, Naoe T, et al. Phase 3, open-label, randomized study of Gilteritinib and Azacitidine vs. Azacitidine for newly diagnosed FLT3-mutated acute myeloid leukemia in patients ineligible for intensive induction chemotherapy. Blood. 2021;138:700.
Daver N, Perl AE, Maly J, Levis M, Ritchie E, Litzow MR, et al. Venetoclax in combination with gilteritinib demonstrates molecular clearance of FLT3 mutation in relapsed/Refractory FLT3-mutated acute myeloid leukemia. Blood. 2021;138:691.
Short NJ, DiNardo CD, Daver N, Nguyen D, Yilmaz M, Kadia TM, et al. A triplet combination of Azacitidine, Venetoclax and Gilteritinib for patients with FLT3-mutated acute myeloid leukemia: results from a Phase I/II Study. Blood. 2021;138:696.
Altman JK, Bhatnagar B, Abedin S, Przespolewski A, Patel PA, Schiller GJ, et al. Gilteritinib can be safely combined with Atezolizumab for the treatment of relapsed or refractory FLT3-mutated AML: Results of a Phase 1 Study. Blood. 2021;138:2343.
Acknowledgements
We would like to thank Astellas for their financial support enabling e-CRF. Astellas was not involved in data collection, analysis, or in the writing of this article.
Author information
Authors and Affiliations
Contributions
Conceptualization, P.Y.D.; Methodology, P.Y.D., E.B.; Patients care, P.Y.D., E.R., S.B., M.A.H., M.H., Y.D., C.B., C.P., J.L., C.O., A.B., F.P., P.P., T.M., M.U., J.F., P.T., T.C., E.J., C.H., E.T., A.V., S.H., M.L.C., M.C., S.C., I.V., M.W., S.C., G.G., R.G., H.D., E.G., K.L., A.M., A.S., A.P., H.D., C.R.; Collected the data, A.M., M.C.B.; Analyzed data, P.Y.D., E.B.; Writing—original draft, P.Y.D.; Revised the manuscript, C.R., J.L., P.P., M.H., C.O., R.G.; Review and editing, P.Y.D., E.B., C.R.; Funding acquisition, P.Y.D., A.M.
Corresponding author
Ethics declarations
Competing interests
Pierre-Yves Dumas: Daiichi-Sankyo, Jazz Pharmaceutical, Astellas, Abbvie, Celgene, Janssen. Emmanuel Raffoux: Daiichi-Sankyo, Astellas, Abbvie, Celgene, Pfizer. Emilie Berard: no competing interest. Sarah Bertoli: Jazz Pharmaceuticals, Daiichi-Sankyo, Sanofi, Astellas and BMS. Marie-Anne Hospital: no competing interest. Maël Heiblig: Astellas, Pfizer, Abbvie, Jazz Pharmaceuticals, Servier. Yohann Desbrosses: Jazz Pharmaceuticals, Abbvie, Celgene, Novartis. Caroline Bonmati: no competing interest. Cécile Pautas: Abbvie, BMS. Juliette Lambert: Pfizer, Astellas, Abbvie. Corentin Orvain: Novartis. Anne Banos: no competing interest. Florence Pasquier: no competing interest. Pierre Peterlin: Daiichi-Sankyo, Jazz Pharmaceutical, Astellas, Abbvie, BMS. Tony Marchand: Jazz Pharmaceutical, Servier. Madalina Uzunov: no competing interest. Jamilé Frayfer: no competing interest. Pascal Turlure: Daiichi-Sankyo. Thomas Cluzeau: Astellas, Novartis and institution: Novartis, Astellas, Arog. Eric Jourdan: Novartis, Abbvie, BMS. Chantal Himberlin: no competing interest. Emmanuelle Tavernier: Abbvie, BMS. Alban Villate: no competing interest. Stephanie Haiat: no competing interest. Marie-Lorraine Chretien: no competing interest. Martin Carre: Astellas, BMS, Jazz pharmaceutical. Sylvain Chantepie: no competing interest. Ioana Vaida: no competing interest. Mathieu Wemeau: Abbvie, AOP Orphan, BMS, Gilead, Novartis. Safia Chebrek: no competing interest. Gaelle Guillerm: no competing interest. Romain Guièze: Abbvie, Janssen, Beigene, Astrazeneca, Roche, Amgen.. Houria Debarri: no competing interest. Eve Gehlkopf: no competing interest. Kamel Laribi: Outside this work, KL received Grants from Novartis, Takeda, Jansen, Abbvie, and personal fees from Novartis, Takeda, Abbvie, Iqone, Astra Zeneca, and Beigene.. Ambroise Marcais: no competing interest. Alberto Santagostino: no competing interest. Marie-Christine Béné: no competing interest. Ariane Mineur: no competing interest. Arnaud Pigneux: Grant/Research Support: Astellas, Roche; Speaker’s Bureau: Astellas, AbbVie, Gilead, Pfizer, Roche, Sanofi; Consultant: Jazz, AbbVie, Agios, BMS, Gilead, Novartis, Pfizer, Roche, Takeda. Hervé Dombret: Honoraria/consulting: Abbvie, Amgen, Astellas, Celgene-BMS, Daiichi Sankyo, Incyte,. Jazz Pharmaceuticals, Pfizer, Servier; research funding: Amgen, Astellas, Celgene-BMS, Incyte, Jazz Pharmaceuticals, Pfizer. Christian Récher: Research grants from AbbVie, Amgen, Novartis, BMS-Celgene, Jazz Pharmaceuticals, Agios, Chugai, MaaT Pharma, Astellas, Roche, Daiichi-Sankyo and Iqvia; an advisory role for AbbVie, Janssen, Jazz Pharmaceuticals, Novartis, Celgene, Otsuka, Astellas, Daiichi-Sankyo, Macrogenics, Pfizer. Roche, Servier and Takeda.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Dumas, PY., Raffoux, E., Bérard, E. et al. Gilteritinib activity in refractory or relapsed FLT3-mutated acute myeloid leukemia patients previously treated by intensive chemotherapy and midostaurin: a study from the French AML Intergroup ALFA/FILO. Leukemia 37, 91–101 (2023). https://doi.org/10.1038/s41375-022-01742-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41375-022-01742-7