Abstract
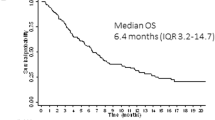
The prospective randomized, placebo-controlled CALGB 10603/RATIFY trial (Alliance) demonstrated a statistically significant overall survival benefit from the addition of midostaurin to standard frontline chemotherapy in a genotypically-defined subgroup of 717 patients with FLT3-mutant acute myeloid leukemia (AML). The risk of death was reduced by 22% on the midostaurin-containing arm. In this post hoc analysis, we analyzed the cumulative incidence of relapse (CIR) on this study and also evaluated the impact of 12 4-week cycles of maintenance therapy. CIR analyses treated relapses and AML deaths as events, deaths from other causes as competing risks, and survivors in remission were censored. CIR was improved on the midostaurin arm (HR = 0.71 (95% CI, 0.54–0.93); p = 0.01), both overall and within European LeukemiaNet 2017 risk classification subsets when post-transplant events were considered in the analysis as events. However, when transplantation was considered as a competing risk, there was overall no significant difference between the risks of relapse on the two randomized arms. Patients still in remission after consolidation with high-dose cytarabine entered the maintenance phase, continuing with either midostaurin or placebo. Analyses were inconclusive in quantifying the impact of the maintenance phase on the overall outcome. In summary, midostaurin reduces the CIR.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Nakao M, Yokota S, Iwai T, Kaneko H, Horiike S, Kashima K, et al. Internal tandem duplication of the flt3 gene found in acute myeloid leukemia. Leukemia. 1996;10:1911–8.
Kottaridis PD, Gale RE, Frew ME, Harrison G, Langabeer SE, Belton AA, et al. The presence of a FLT3 internal tandem duplication in patients with acute myeloid leukemia (AML) adds important prognostic information to cytogenetic risk group and response to the first cycle of chemotherapy: analysis of 854 patients from the United Kingdom Medical Research Council AML 10 and 12 trials. Blood. 2001;98:1752–9.
Nagel G, Weber D, Fromm E, Erhardt S, Lübbert M, Fiedler W, et al. Epidemiological, genetic, and clinical characterization by age of newly diagnosed acute myeloid leukemia based on an academic population-based registry study (AMLSG BiO). Ann Hematol. 2017;96:1993–2003.
Thiede C, Steudel C, Mohr B, Schaich M, Schäkel U, Platzbecker U, et al. Analysis of FLT3-activating mutations in 979 patients with acute myelogenous leukemia: association with FAB subtypes and identification of subgroups with poor prognosis. Blood. 2002;99:4326–35.
Whitman SP, Archer KJ, Feng L, Baldus C, Becknell B, Carlson BD, et al. Absence of the wild-type allele predicts poor prognosis in adult de novo acute myeloid leukemia with normal cytogenetics and the internal tandem duplication of FLT3: a Cancer and Leukemia Group B study. Cancer Res. 2001;61:7233–9.
Mead AJ, Linch DC, Hills RK, Wheatley K, Burnett AK, Gale RE. FLT3 tyrosine kinase domain mutations are biologically distinct from and have a significantly more favorable prognosis than FLT3 internal tandem duplications in patients with acute myeloid leukemia. Blood. 2007;110:1262–70.
Whitman SP, Ruppert AS, Radmacher MD, Mrózek K, Paschka P, Langer C, et al. FLT3 D835/I836 mutations are associated with poor disease-free survival and a distinct gene-expression signature among younger adults with de novo cytogenetically normal acute myeloid leukemia lacking FLT3 internal tandem duplications. Blood. 2008;111:1552–9.
Weisberg E, Boulton C, Kelly LM, Manley P, Fabbro D, Meyer T, et al. Inhibition of mutant FLT3 receptors in leukemia cells by the small molecule tyrosine kinase inhibitor PKC412. Cancer Cell. 2002;1:433–43.
Pratz KW, Levis MJ. Bench to bedside targeting of FLT3 in acute leukemia. Curr Drug Targets. 2010;11:781–9.
Levis M, Pham R, Smith BD, Small D. In vitro studies of a FLT3 inhibitor combined with chemotherapy: sequence of administration is important to achieve synergistic cytotoxic effects. Blood. 2004;104:1145–50.
Perl AE, Altman JK, Cortes J, Smith C, Litzow M, Baer MR, et al. Selective inhibition of FLT3 by gilteritinib in relapsed or refractory acute myeloid leukaemia: a multicentre, first-in-human, open-label, phase 1-2 study. Lancet Oncol. 2017;18:1061–75.
Cortes J, Perl AE, Döhner H, Kantarjian H, Martinelli G, Kovacsovics T, et al. Quizartinib, an FLT3 inhibitor, as monotherapy in patients with relapsed or refractory acute myeloid leukaemia: an open-label, multicentre, single-arm, phase 2 trial. Lancet Oncol. 2018;19:889–903.
Stone RM, Manley PW, Larson RA, Capdeville R. Midostaurin: its odyssey from discovery to approval for treating acute myeloid leukemia and advanced systemic mastocytosis. Blood Adv. 2018;2:444–53. 27
Stone RM, DeAngelo DJ, Klimek V, Galinsky I, Estey E, Nimer SD, et al. Patients with acute myeloid leukemia and an activating mutation in FLT3 respond to a small-molecule FLT3 tyrosine kinase inhibitor, PKC412. Blood. 2005;105:54–60.
Stone RM, Fischer T, Paquette R, Schiller G, Schiffer CA, Ehninger G, et al. Phase IB study of the FLT3 kinase inhibitor midostaurin with chemotherapy in younger newly diagnosed adult patients with acute myeloid leukemia. Leukemia. 2012;26:2061–8.
Fischer T, Stone RM, DeAngelo DJ, Galinsky I, Estey E, Lanza C, et al. Phase IIB trial of oral midostaurin (PKC412), the FMS-like tyrosine kinase 3 receptor (FLT3) and multi-targeted kinase inhibitor, in patients with acute myeloid leukemia and high-risk myelodysplastic syndrome with either wild-type or mutated FLT3. J Clin Oncol. 2010;28:4339–45.
Stone RM, Mandrekar SJ, Sanford BL, Laumann K, Geyer S, Bloomfield CD, et al. Midostaurin plus chemotherapy for acute myeloid leukemia with a FLT3 mutation. N. Engl J Med. 2017;377:454–64.
Tzogani K, Yu Y, Meulendijks D, Herberts C, Hennik P, Verheijen R, et al. European Medicines Agency review of midostaurin (Rydapt) for the treatment of adult patients with acute myeloid leukaemia and systemic mastocytosis. ESMO Open. 2019;4:e000606. https://doi.org/10.1136/esmoopen-2019-000606
Schlenk RF, Kayser S, Bullinger L, Kobbe G, Casper J, Ringhoffer M, et al. Differential impact of allelic ratio and insertion site in FLT3-ITD-positive AML with respect to allogeneic transplantation. Blood. 2014;124:3441–9.
Döhner H, Estey E, Grimwade D, Amadori S, Appelbaum FR, Büchner T, et al. Diagnosis and management of AML in adults: 2017 ELN recommendations from an international expert panel. Blood. 2017;129:424–47.
Döhner K, Thiede C, Jahn N, Panina E, Gambietz A, Larson RA, et al. Impact of NPM1/FLT3-ITD genotypes defined by the 2017 European LeukemiaNet in patients with acute myeloid leukemia. Blood. 2020;135:371–80.
Voso MT, Larson RA, Jones D, Marcucci G, Prior T, Krauter J, et al. Midostaurin in patients with acute myeloid leukemia and FLT3-TKD mutations: a sub-analysis from the RATIFY trial. Blood Adv. 2020;4:4945–54.
Thiede C, Prior TW, Lo-Coco F, Krauter J, Barragán E, Nomdedeu J, et al. FLT3 Mutation Assay Laboratory Cross Validation: Results from the CALGB 10603/RATIFY (Alliance) trial in patients with newly diagnosed FLT3-mutated acute myeloid leukemia. Blood. 2018;132:abstract 2800.
Levis M, Shi W, Chang K, Laing C, Pollner R, Gocke C, et al. FLT3 inhibitors added to induction therapy induce deeper remissions. Blood. 2020;135:75–78.
Schlenk RF, Weber D, Fiedler W, Salih HR, Wulf G, Salwender H, et al. Midostaurin added to chemotherapy and continued single-agent maintenance therapy in acute myeloid leukemia with FLT3-ITD. Blood. 2019;133:840–51.
Wei AH, Döhner H, Pocock C, Montesinos P, Afanasyev B, Dombret H, et al. Oral azacitidine maintenance therapy for acute myeloid leukemia in first remission. N. Engl J Med. 2020;383:2526–37.
Mathew NR, Baumgartner F, Braun L, O’Sullivan D, Thomas S, Waterhouse M, et al. Sorafenib promotes graft-versus-leukemia activity in mice and humans through IL-15 production in FLT3-ITD-mutant leukemia cells. Nat Med. 2018;24:282–91.
Metzelder SK, Schroeder T, Lübbert M, Ditschkowski M, Götze K, Scholl S, et al. Long-term survival of sorafenib-treated FLT3-ITD-positive acute myeloid leukaemia patients relapsing after allogeneic stem cell transplantation. Eur J Cancer. 2017;86:233–9.
Burchert A, Bug G, Fritz LV, Finke J, Stelljes M, Röllig C, et al. Sorafenib maintenance after allogeneic hematopoietic stem cell transplantation for acute myeloid leukemia with FLT3-internal tandem duplication mutation (SORMAIN). J Clin Oncol. 2020;38:2993–3002.
Maziarz RT, Levis M, Patnaik MM, Scott BL, Mohan SR, Deol A, et al. Midostaurin after allogeneic stem cell transplant in patients with FLT3-internal tandem duplication-positive acute myeloid leukemia. Bone Marrow Transplant. 2020, https://doi.org/10.1038/s41409-020-01153-1
Perl AE, Martinelli G, Cortes JE, Neubauer A, Berman E, Paolini S, et al. Gilteritinib or chemotherapy for relapsed or refractory FLT3-mutated AML. N. Engl J Med. 2019;381:1728–40.
Cortes JE, Khaled S, Martinelli G, Perl AE, Ganguly S, Russell N, et al. Quizartinib versus salvage chemotherapy in relapsed or refractory FLT3-ITD acute myeloid leukaemia (QuANTUM-R): a multicentre, randomised, controlled, open-label, phase 3 trial. Lancet Oncol. 2019;20:984–97.
Acknowledgements
We thank the patients who participated and their families, the clinical and laboratory research staff members of multiple international cooperative groups, CTEP of the NCI, and Novartis Pharmaceuticals. We acknowledge Dr. Francesco Lo-Coco’s and Dr. Clara D. Bloomfield’s key roles in the design and completion of this study. CALGB is now part of the Alliance for Clinical Trials in Oncology. Research reported in this publication was supported by the National Cancer Institute of the National Institutes of Health under award numbers U10CA180821, U10CA180882, U24CA198171 (to the Alliance for Clinical Trials in Oncology), U10CA032291, U10CA041287, U10CA077651, U10CA077658, U10CA180791, U10CA180820 (ECOG-ACRIN), UG1CA233290, U10CA180836, U10CA180850, U10CA180863 (CCTG), U10CA180867, U10CA180888 (SWOG), and UG1CA233338 [https://acknowledgments.alliancefound.org]. This study was also supported in part by funds from Novartis. Clinicaltrials.gov Identifier number: NCT00651261.
Author information
Authors and Affiliations
Contributions
RAL and RMS designed the study, performed research, collected, assembled, analyzed, and interpreted data, and wrote the manuscript; SJM, LJH, BLS, KL, SG, and IGathmann performed statistical analyses; CDB, CT, TWP, KD, GM, MTV, RBK, IGallinsky, AHW, JS, MAS, JMB, TdW, DN, FRA, BCM, MST, JK, RFS, AG, HS, GE, SA, and HD collected, assembled, analyzed, and interpreted data, and edited the manuscript. All authors approved the final version of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
RAL has acted as a consultant or advisor to Novartis, Amgen, Ariad/Takeda, Astellas, Celgene/BMS, CVS/Caremark, Epizyme, and MorphoSys, and has received clinical research support from Novartis, Astellas, Celgene, Cellectis, Daiichi Sankyo, Forty Seven, Rafael Pharmaceuticals, and royalties from UpToDate. SJM has acted as a consultant or advisor for Pfizer and Pique Therapeutics, and has another relationship with BeiGene. CT is the Chief Executive Officer and a co-owner of AgenDix, a company performing molecular diagnostics, and has acted as a consultant or advisor for Novartis and Astellas, and has received clinical research support from Bayer. KD has acted as a consultant or advisor for Astellas, Celgene, Daiichi Sankyo, Janssen, Novartis, and Roche and has received clinical research support from Astex, Celgene, and Novartis. GM, RBK, DN, and HS have acted as consultants or advisors for Novartis. AHW has acted as a consultant or advisor for Novartis, Astellas, Pfizer, MacroGenics, AbbVie, Genentech, Servier, Celgene, Amgen, Astra Zeneca, and Janssen, and is a member of the speakers bureau for AbbVie/Genentech and Novartis, and has received research funding from Novartis, Celgene, AbbVie, Servier, Astra Zeneca, and Amgen, and is a former employee of the Walter and Eliza Hall Institute and receives a fraction of its royalty stream related to venetoclax. JS has acted as a consultant or advisor for Pfizer, Daiichi Sankyo, AbbVie, Novartis, Astellas, and Roche and is a member of the speakers bureau for Novartis, Pfizer, Daiichi Sankyo, and AbbVie. MAS has acted as a consultant or advisor for Teva Pharmaceutical Industries, Daiichi Sankyo, Orsenix, AbbVie, Novartis, and Pfizer. TdW has acted as a consultant or advisor for Novartis, Celgene, Johnson & Johnson, and Incyte and has received clinical research support from Novartis, Celgene, and Johnson & Johnson. BCM has acted as a consultant or advisor for Celgene, Novartis, and Astellas and is currently employed by Roche/Genentech. MST has acted as a consultant or advisor for AbbVie, BioLineRx, Daiichi Sankyo, Orsenix, KAHR Medical, Rigel Pharmaceuticals, Nohla, Delta Fly Pharma, Tetraphase, Oncolyze, and Jazz Pharmaceuticals, and has received clinical research funding from AbbVie, Cellerant Therapeutics, Orsenix, ADC Therapeutics, and BioSight, and has received royalties from UpToDate. JK has acted as a consultant or advisor for Amgen, Astellas, Bristol-Myers Squibb, Daiichi Sankyo, and Pfizer. AG has received clinical research support from Novartis. RFS has acted as a consultant or advisor for Pfizer and Daiichi Sankyo, is a member of the speakers bureau for Novartis, Pfizer, and Daiichi Sankyo, and has received clinical research funding from Pfizer, Daiichi Sankyo, PharmaMar, Astra Zeneca, and Roche. SA has acted as a consultant or advisor for Novartis and Daiichi Sankyo. IGathmann is an employee of Novartis. HD has acted as a consultant or advisor for AbbVie, Agios, Amgen, Astellas, Astex Pharmaceuticals, Celgene, Helsinn, Janssen, Jazz Pharmaceuticals, Novartis, Oxford Biomedicals, and Roche, and has received institutional research support from Amgen, AROG Pharmaceuticals, Bristol-Myers Squibb, Celgene, Jazz Pharmaceuticals, Novartis, Pfizer, and Sunesis. RMS has acted as a consultant or advisor for AbbVie, Actinium, and Agios, has received personal fees from Amgen, Argenx, AROG, Astellas, Astra Zeneca, BioLineRx, Celgene, Cornerstone, Daiichi Sankyo, Fujifilm, Jazz Pharmaceuticals, MacroGenics, Novartis, Ono/Theradex Oncology, Orsenix, Otsuka/Astex, Pfizer, Roche, Stemline Therapeutics, Takeda, and Trovagene, and has received institutional research support from AbbVie, Agios, AROG, and Novartis. The remaining authors declare no competing financial interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Larson, R.A., Mandrekar, S.J., Huebner, L.J. et al. Midostaurin reduces relapse in FLT3-mutant acute myeloid leukemia: the Alliance CALGB 10603/RATIFY trial. Leukemia 35, 2539–2551 (2021). https://doi.org/10.1038/s41375-021-01179-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41375-021-01179-4
This article is cited by
-
Biomarkers as targets for CAR-T/NK cell therapy in AML
Biomarker Research (2023)
-
Acute myeloid leukemia: from NGS, through scRNA-seq, to CAR-T. dissect cancer heterogeneity and tailor the treatment
Journal of Experimental & Clinical Cancer Research (2023)
-
Autologous stem cell transplantation in adult patients with intermediate-risk acute myeloid leukemia in first complete remission and no detectable minimal residual disease. A comparative retrospective study with haploidentical transplants of the global committee and the ALWP of the EBMT
Bone Marrow Transplantation (2023)
-
Treatment of older adults with FLT3-mutated AML: Emerging paradigms and the role of frontline FLT3 inhibitors
Blood Cancer Journal (2023)
-
Perspectives and challenges of small molecule inhibitor therapy for FLT3-mutated acute myeloid leukemia
Annals of Hematology (2023)