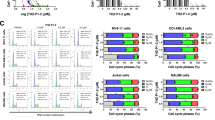
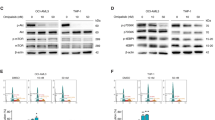
Abstract
Acute myeloid leukemia (AML) is a heterogeneous group of aggressive hematological malignancies commonly associated with treatment resistance, high risk of relapse, and mitochondrial dysregulation. We identified six mitochondria-affecting compounds (PS compounds) that exhibit selective cytotoxicity against AML cells in vitro. Structure-activity relationship studies identified six analogs from two original scaffolds that had over an order of magnitude difference between LD50 in AML and healthy peripheral blood mononuclear cells. Mechanistically, all hit compounds reduced ATP and selectively impaired both basal and ATP-linked oxygen consumption in leukemic cells. Compounds derived from PS127 significantly upregulated production of reactive oxygen species (ROS) in AML cells and triggered ferroptotic, necroptotic, and/or apoptotic cell death in AML cell lines and refractory/relapsed AML primary samples. These compounds exhibited synergy with several anti-leukemia agents in AML, acute lymphoblastic leukemia (ALL), or chronic myelogenous leukemia (CML). Pilot in vivo efficacy studies indicate anti-leukemic efficacy in a MOLM14/GFP/LUC xenograft model, including extended survival in mice injected with leukemic cells pre-treated with PS127B or PS127E and in mice treated with PS127E at a dose of 5 mg/kg. These compounds are promising leads for development of future combinatorial therapeutic approaches for mitochondria-driven hematologic malignancies such as AML, ALL, and CML.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
Data availability
The data in this study that support the findings of this study are available from the corresponding author upon reasonable request.
Code availability
The code that supports the findings of this study are available from the corresponding author upon reasonable request.
References
Weinberg F, Hamanaka R, Wheaton WW, Weinberg S, Joseph J, Lopez M, et al. Mitochondrial metabolism and ROS generation are essential for Kras-mediated tumorigenicity. Proc Natl Acad Sci USA. 2010;107:8788–93.
Wise DR, Thompson CB. Glutamine addiction: A new therapeutic target in cancer. Trends Biochem Sci. 2010;35:427–33.
Battogtokh G, Cho YY, Lee JY, Lee HS, Kang HC. Mitochondrial-targeting anticancer agent conjugates and nanocarrier systems for cancer treatment. Front Pharm. 2018;9:922.
Neagu M, Constantin C, Popescu ID, Zipeto D, Tzanakakis G, Nikitovic D, et al. Inflammation and metabolism in cancer cell-mitochondria key player. Front Oncol. 2019;9:348.
Macleod K. Mitophagy and mitochondrial dysfunction in cancer. Annu Rev Cancer Biol. 2020;4:41–60.
Moro L. Mitochondrial dysfunction in aging and cancer. J Clin Med. 2019;8:1983.
Zanotto-Filho A, Delgado-Cañedo A, Schröder R, Becker M, Klamt F, Moreira JC. The pharmacological NFkappaB inhibitors BAY117082 and MG132 induce cell arrest and apoptosis in leukemia cells through ROS-mitochondria pathway activation. Cancer Lett. 2010;288:192–203.
Zunino SJ, Storms DH. Resveratrol-induced apoptosis is enhanced in acute lymphoblastic leukemia cells by modulation of the mitochondrial permeability transition pore. Cancer Lett. 2006;240:123–34.
Baccelli I, Gareau Y, Lehnertz B, Gingras S, Spinella JF, Corneau S, et al. Mubritinib targets the electron transport chain complex I and reveals the landscape of OXPHOS dependency in acute myeloid leukemia. Cancer Cell. 2019;36:84–99.e8.
Sillar JR, Germon ZP, DeIuliis GN, Dun MD. The role of reactive oxygen species in acute myeloid leukaemia. Int J Mol Sci. 2019;20:6003.
Kreitz J, Schönfeld C, Seibert M, Stolp V, Alshamleh I, Oellerich T, et al. Metabolic plasticity of acute myeloid leukemia. Cells. 2019;8:805.
Döhner H, Estey EH, Amadori S, Appelbaum FR, Büchner T, Burnett AK, et al. Diagnosis and management of acute myeloid leukemia in adults: Recommendations from an international expert panel, on behalf of the European LeukemiaNet. Blood 2010;115:453–74.
Panina SB, Baran N, Brasil da Costa FH, Konopleva M, Kirienko NV. A mechanism for increased sensitivity of acute myeloid leukemia to mitotoxic drugs. Cell Death Dis. 2019;10:617.
Panina SB, Pei J, Baran N, Konopleva M, Kirienko NV. Utilizing synergistic potential of mitochondria-targeting drugs for leukemia therapy. Front Oncol. 2020;10:435.
Wang Y, Liu HH, Cao YT, Zhang LL, Huang F, Yi C. The role of mitochondrial dynamics and mitophagy in carcinogenesis, metastasis and therapy. Front Cell Dev Biol. 2020;8:413.
Vara-Perez M, Felipe-Abrio B, Agostinis P. Mitophagy in cancer: A tale of adaptation. Cells. 2019;8:493.
Yan C, Li TS. Dual role of mitophagy in cancer drug resistance. Anticancer Res. 2018;38:617–21.
Biel TG, Rao VA. Mitochondrial dysfunction activates lysosomal-dependent mitophagy selectively in cancer cells. Oncotarget 2018;9:995–1011.
Morad SAF, MacDougall MR, Abdelmageed N, Kao LP, Feith DJ, Tan SF, et al. Pivotal role of mitophagy in response of acute myelogenous leukemia to a ceramide-tamoxifen-containing drug regimen. Exp Cell Res. 2019;381:256–64.
Tjahjono E, Pei J, Revtovich AV, Liu T-JE, Swadi A, Hancu MC, et al. Mitochondria-affecting small molecules ameliorate proteostasis defects associated with neurodegenerative disease. Sci Rep. 2021;11:17733.
Molina JR, Sun Y, Protopopova M, Gera S, Bandi M, Bristow C, et al. An inhibitor of oxidative phosphorylation exploits cancer vulnerability. Nat Med. 2018;24:1036–46.
Bostrom B, Erdmann G. Cellular pharmacology of 6-mercaptopurine in acute lymphoblastic leukemia. Am J Pediatr Hematol Oncol. 1993;15:80–6.
Tacar O, Sriamornsak P, Dass CR. Doxorubicin: an update on anticancer molecular action, toxicity and novel drug delivery systems. J Pharm Pharm. 2013;65:157–70.
Pei J, Panina SB, Kirienko NV. An automated differential nuclear staining assay for accurate determination of mitocan cytotoxicity. J Vis Exp. 2020;159:e61295.
Ritz C, Baty F, Streibig JC, Gerhard D, Dose-Response Analysis Using R. PLoS One. 2015;10:e0146021.
Ianevski A, He L, Aittokallio T, Tang J. SynergyFinder: a web application for analyzing drug combination dose-response matrix data. Bioinformatics 2017;33:2413–5.
Li N, Ragheb K, Lawler G, Sturgis J, Rajwa B, Melendez JA, et al. Mitochondrial complex I inhibitor rotenone induces apoptosis through enhancing mitochondrial reactive oxygen species production. J Biol Chem. 2003;278:8516–25.
Chaoui D, Faussat AM, Majdak P, Tang R, Perrot JY, Pasco S, et al. JC-1, a sensitive probe for a simultaneous detection of P-glycoprotein activity and apoptosis in leukemic cells. Cytom B Clin Cytom. 2006;70:189–96.
Brookes PS. Mitochondrial H(+) leak and ROS generation: an odd couple. Free Radic Biol Med. 2005;38:12–23.
Kari E, Teppo HR, Haapasaari KM, Kuusisto MEL, Lemma A, Karihtala P, et al. Nuclear factor erythroid 2-related factors 1 and 2 are able to define the worst prognosis group among high-risk diffuse large B cell lymphomas treated with R-CHOEP. J Clin Pathol. 2019;72:316–21.
Evans MJ, Scarpulla RC. NRF-1: A trans-activator of nuclear-encoded respiratory genes in animal cells. Genes Dev. 1990;4:1023–34.
Vélez J, Hail N, Konopleva M, Zeng Z, Kojima K, Samudio I, et al. Mitochondrial uncoupling and the reprograming of intermediary metabolism in leukemia cells. Front Oncol. 2013;3:67.
Mookerjee SA, Brand MD. Measurement and analysis of extracellular acid production to determine Glycolytic rate. J Vis Exp. 2015;106:e53464.
Jantas D, Chwastek J, Grygier B, Lasoń W. Neuroprotective effects of Necrostatin-1 against oxidative stress-induced cell damage: An involvement of Cathepsin D inhibition. Neurotox Res. 2020;37:525–42.
Dixon SJ, Lemberg KM, Lamprecht MR, Skouta R, Zaitsev EM, Gleason CE, et al. Ferroptosis: An iron-dependent form of nonapoptotic cell death. Cell 2012;149:1060–72.
Yao X, Zhang Y, Hao J, Duan HQ, Zhao CX, Sun C, et al. Deferoxamine promotes recovery of traumatic spinal cord injury by inhibiting ferroptosis. Neural Regen Res. 2019;14:532–41.
Dombret H, Gardin C. An update of current treatments for adult acute myeloid leukemia. Blood 2016;127:53–61.
Tamamyan G, Kadia T, Ravandi F, Borthakur G, Cortes J, Jabbour E, et al. Frontline treatment of acute myeloid leukemia in adults. Crit Rev Oncol Hematol. 2017;110:20–34.
Han L, Cavazos A, Baran N, Zhang Q, Kuruvilla VM, Gay JP, et al. Mitochondrial Oxphos as survival mechanism of minimal residual AML cells after induction chemotherapy: Survival benefit By Complex I inhibition with Iacs-010759. Blood 2019;134:5161.
Baran N, Lodi A, Sweeney SR, Renu P, Kuruvilla VM, Cavazos A, et al. Mitochondrial complex I inhibitor Iacs-010759 reverses the NOTCH1-driven metabolic reprogramming in T-ALL via blockade of oxidative phosphorylation: Synergy with chemotherapy and glutaminase inhibition. Blood 2018;132:4020.
Rytelewski M, Harutyunyan K, Baran N, Mallampati S, Zal M, Cavazos A, et al. Inhibition of oxidative phosphorylation reverses bone marrow hypoxia visualized in imageable syngeneic B-ALL Mouse Model. Front Oncol. 2020;10:991.
Castelli G, Pelosi E, Testa U. Emerging therapies for acute myelogenus leukemia patients targeting apoptosis and mitochondrial metabolism. Cancers (Basel). 2019;11:260.
Basak NP, Banerjee S. Mitochondrial dependency in progression of acute myeloid leukemia. Mitochondrion 2015;21:41–8.
Filimonov DA, Lagunin AA, Gloriozova TA, Rudik AV, Druzhilovskii DS, Pogodin PV, et al. Prediction of the Biological Activity Spectra of Organic Compounds Using the Pass Online Web Resource. Chem Heterocycl Compd. 2014;50:444–57.
Bright SA, Byrne AJ, Vandenberghe E, Browne PV, Mcelligott AM, Meegan MJ, et al. Selected nitrostyrene compounds demonstrate potent activity in chronic lymphocytic leukaemia cells, including those with poor prognostic markers. Oncol Rep. 2019;41:3127–36.
Wang YY, Chen YK, Hsu YL, Chiu WC, Tsai CH, Hu SC, et al. Synthetic β-nitrostyrene derivative CYT-Rx20 as inhibitor of oral cancer cell proliferation and tumor growth through glutathione suppression and reactive oxygen species induction. Head Neck. 2017;39:1055–64.
Han K, Jeng EE, Hess GT, Morgens DW, Li A, Bassik MC. Synergistic drug combinations for cancer identified in a CRISPR screen for pairwise genetic interactions. Nat Biotechnol. 2017;35:463–74.
Rowe JM. Therapy of secondary leukemia. Leukemia 2002;16:748–50.
Veelken H, Licht T, Lais A, Köhler G, Mertelsmann R, Schaefer HE, et al. Drug resistance of secondary acute myeloid leukemia with megakaryoblastic features and p190 BCR-ABL rearrangement. Leuk Res. 1998;22:1021–7.
Kim EH, Sohn S, Kwon HJ, Kim SU, Kim MJ, Lee SJ, et al. Sodium selenite induces superoxide-mediated mitochondrial damage and subsequent autophagic cell death in malignant glioma cells. Cancer Res. 2007;67:6314–24.
Yan C, Luo L, Guo CY, Goto S, Urata Y, Shao JH, et al. Doxorubicin-induced mitophagy contributes to drug resistance in cancer stem cells from HCT8 human colorectal cancer cells. Cancer Lett. 2017;388:34–42.
Pei S, Minhajuddin M, Adane B, Khan N, Stevens BM, Mack SC, et al. AMPK/FIS1-Mediated Mitophagy Is Required for Self-Renewal of Human AML Stem Cells. Cell Stem Cell. 2018;23:86–100.e6.
Fay HRS, Dykstra KM, Johnson M, Cronin TL, Lutgen-Dunckley L, Martens BL, et al. Mitophagy Plays a Key Role in the Anti-Leukemic Activity of Autophagy Inhibitors Under Hypoxia in Acute Myeloid Leukemia. Blood 2019;134:1278.
Abo Elwafa R, Gamaleldin M, Ghallab O. The clinical and prognostic significance of FIS1, SPI1, PDCD7 and Ang2 expression levels in acute myeloid leukemia. Cancer Genet. 2019;233-234:84–95.
Dany M, Gencer S, Nganga R, Thomas RJ, Oleinik N, Baron KD, et al. Targeting FLT3-ITD signaling mediates ceramide-dependent mitophagy and attenuates drug resistance in AML. Blood 2016;128:1944–58.
Daver N, Schlenk RF, Russell NH, Levis MJ. Targeting FLT3 mutations in AML: Review of current knowledge and evidence. Leukemia 2019;33:299–312.
Yao N, Wang C, Hu N, Li Y, Liu M, Lei Y, et al. Inhibition of PINK1/Parkin-dependent mitophagy sensitizes multidrug-resistant cancer cells to B5G1, a new betulinic acid analog. Cell Death Dis. 2019;10:232.
Kameyama K, Motoyama K, Tanaka N, Yamashita Y, Higashi T, Arima H. Induction of mitophagy-mediated antitumor activity with folate-appended methyl-β-cyclodextrin. Int J Nanomed. 2017;12:3433–46.
Basit F, van Oppen LM, Schöckel L, Bossenbroek HM, van Emst-de Vries SE, Hermeling JC, et al. Mitochondrial complex I inhibition triggers a mitophagy-dependent ROS increase leading to necroptosis and ferroptosis in melanoma cells. Cell Death Dis. 2017;8:e2716.
Acknowledgements
The study was supported by the CPRIT grant RR150044 and NIH NIGMS grant R35GM129294 to NVK and NIH NCI grants R01CA231364 and P50 CA100632 to MK. Computer-aided estimation of which regions of the chemical scaffold are relevant for cytotoxicity (L.A.S. and V.V.P.) was performed in the framework of the Russian Federation Fundamental Research Program for the long-term period for 2021–2030 (No. 122030100170-5).
Author information
Authors and Affiliations
Contributions
SBP and JP performed the majority of the experiments and wrote the first draft of the manuscript. NB assisted with ATP measurements, conducted seahorse experiments, flow cytometry experiments, mouse studies, and contributed to manuscript writing and editing. ET performed some of the experiments and contributed to manuscript writing and editing. SP assisted with mouse experiments and provided patient samples, GA, and SNK provided healthy bone marrow and patient samples. LAS and VVP performed cheminformatic analysis. MK and NK performed overall design of the study, acquired funding, and contributed to data analysis and manuscript writing and editing. All authors reviewed the manuscript before submission.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Panina, S.B., Pei, J., Baran, N. et al. Novel mitochondria-targeting compounds selectively kill human leukemia cells. Leukemia 36, 2009–2021 (2022). https://doi.org/10.1038/s41375-022-01614-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41375-022-01614-0