Abstract
Immunoglobulin light chain (AL) amyloidosis is caused by a small, minimally proliferating B-cell/plasma-cell clone secreting a patient-unique, aggregation-prone, toxic light chain (LC). The pathogenicity of LCs is encrypted in their sequence, yet molecular determinants of amyloidogenesis are poorly understood. Higher rates of N-glycosylation among clonal κ LCs from patients with AL amyloidosis compared to other monoclonal gammopathies indicate that this post-translational modification is associated with a higher risk of developing AL amyloidosis. Here, we exploited LC sequence information from previously published amyloidogenic and control clonal LCs and from a series of 220 patients with AL amyloidosis or multiple myeloma followed at our Institutions to define sequence and spatial features of N-glycosylation, combining bioinformatics, biochemical, proteomics, structural and genetic analyses. We found peculiar sequence and spatial pattern of N-glycosylation in amyloidogenic κ LCs, with most of the N-glycosylation sites laying in the framework region 3, particularly within the E strand, and consisting mainly of the NFT sequon, setting them apart with respect to non-amyloidogenic clonal LCs. Our data further support a potential role of N-glycosylation in determining the pathogenic behavior of a subset of amyloidogenic LCs and may help refine current N-glycosylation-based prognostic assessments for patients with monoclonal gammopathies.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
The data generated in this study are available within the article and its Supplementary Data Files. LC sequences have been deposited to GenBank (MZ595009-MZ595094, OM885091-OM885224).
References
Merlini G, Dispenzieri A, Sanchorawala V, Schonland SO, Palladini G, Hawkins PN, et al. Systemic immunoglobulin light chain amyloidosis. Nat Rev Dis Prim. 2018;4:38.
Comenzo RL, Zhang Y, Martinez C, Osman K, Herrera GA. The tropism of organ involvement in primary systemic amyloidosis: contributions of Ig V(L) germ line gene use and clonal plasma cell burden. Blood. 2001;98:714–20.
Abraham RS, Geyer SM, Price-Troska TL, Allmer C, Kyle RA, Gertz MA, et al. Immunoglobulin light chain variable (V) region genes influence clinical presentation and outcome in light chain-associated amyloidosis (AL). Blood. 2003;101:3801–8.
Perfetti V, Casarini S, Palladini G, Vignarelli MC, Klersy C, Diegoli M, et al. Analysis of V(lambda)-J(lambda) expression in plasma cells from primary (AL) amyloidosis and normal bone marrow identifies 3r (lambdaIII) as a new amyloid-associated germline gene segment. Blood. 2002;100:948–53.
Prokaeva T, Spencer B, Kaut M, Ozonoff A, Doros G, Connors LH, et al. Soft tissue, joint, and bone manifestations of AL amyloidosis: clinical presentation, molecular features, and survival. Arthritis Rheum. 2007;56:3858–68.
Perfetti V, Palladini G, Casarini S, Navazza V, Rognoni P, Obici L, et al. The repertoire of lambda light chains causing predominant amyloid heart involvement and identification of a preferentially involved germline gene, IGLV1-44. Blood. 2012;119:144–50.
Bodi K, Prokaeva T, Spencer B, Eberhard M, Connors LH, Seldin DC. AL-Base: a visual platform analysis tool for the study of amyloidogenic immunoglobulin light chain sequences. Amyloid. 2009;16:1–8.
Dwulet FE, O’Connor TP, Benson MD. Polymorphism in a kappa I primary (AL) amyloid protein (BAN). Mol Immunol. 1986;23:73–8.
Stevens FJ. Four structural risk factors identify most fibril-forming kappa light chains. Amyloid. 2000;7:200–11.
Omtvedt LA, Bailey D, Renouf DV, Davies MJ, Paramonov NA, Haavik S, et al. Glycosylation of immunoglobulin light chains associated with amyloidosis. Amyloid. 2000;7:227–44.
Karimi M, Sletten K, Westermark P. Biclonal systemic AL-amyloidosis with one glycosylated and one nonglycosylated AL-protein. Scand J Immunol. 2003;57:319–23.
Myran T, Husby G, Kyle RA, Sletten K. The amino acid sequence of a glycosylated AL-chain from a patient with primary amyloidosis. Amyloid. 2004;11:109–12.
Connors LH, Jiang Y, Budnik M, Theberge R, Prokaeva T, Bodi KL, et al. Heterogeneity in primary structure, post-translational modifications, and germline gene usage of nine full-length amyloidogenic kappa1 immunoglobulin light chains. Biochemistry. 2007;46:14259–71.
Kumar S, Murray D, Dasari S, Milani P, Barnidge D, Madden B, et al. Assay to rapidly screen for immunoglobulin light chain glycosylation: a potential path to earlier AL diagnosis for a subset of patients. Leukemia. 2019;33:254–7.
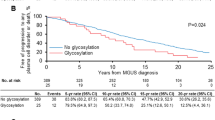
Dispenzieri A, Larson DR, Rajkumar SV, Kyle RA, Kumar SK, Kourelis T, et al. N-glycosylation of monoclonal light chains on routine MASS-FIX testing is a risk factor for MGUS progression. Leukemia. 2020;34:2749–53.
Mellors PW, Dasari S, Kohlhagen MC, Kourelis T, Go RS, Muchtar E, et al. MASS-FIX for the detection of monoclonal proteins and light chain N-glycosylation in routine clinical practice: a cross-sectional study of 6315 patients. Blood Cancer J. 2021;11:50.
Milani P, Murray DL, Barnidge DR, Kohlhagen MC, Mills JR, Merlini G, et al. The utility of MASS-FIX to detect and monitor monoclonal proteins in the clinic. Am J Hematol. 2017;92:772–9.
Kourelis T, Murray DL, Dasari S, Kumar S, Barnidge D, Madden B, et al. MASS-FIX may allow identification of patients at risk for light chain amyloidosis before the onset of symptoms. Am J Hematol. 2018;93:E368–70.
Alameda D, Goicoechea I, Vicari M, Arriazu E, Nevone A, Rodriguez S, et al. Tumor cells in light-chain amyloidosis and myeloma show distinct transcriptional rewiring of normal plasma cell development. Blood. 2021;138:1583–9.
Gupta R, Brunak S. Prediction of glycosylation across the human proteome and the correlation to protein function. Pac Symp Biocomput. 2002;7:310–22.
Lavatelli F, Mazzini G, Ricagno S, Iavarone F, Rognoni P, Milani P, et al. Mass spectrometry characterization of light chain fragmentation sites in cardiac AL amyloidosis: insights into the timing of proteolysis. J Biol Chem. 2020;295:16572–84.
van de Bovenkamp FS, Derksen NIL, Ooijevaar-de Heer P, van Schie KA, Kruithof S, Berkowska MA, et al. Adaptive antibody diversification through N-linked glycosylation of the immunoglobulin variable region. Proc Natl Acad Sci USA. 2018;115:1901–6.
Chicco D, Jurman G. The advantages of the Matthews correlation coefficient (MCC) over F1 score and accuracy in binary classification evaluation. BMC Genom. 2020;21:6.
Imperiali B, Hendrickson TL. Asparagine-linked glycosylation: specificity and function of oligosaccharyl transferase. Bioorg Med Chem. 1995;3:1565–78.
Maley F, Trimble RB, Tarentino AL, Plummer TH Jr. Characterization of glycoproteins and their associated oligosaccharides through the use of endoglycosidases. Anal Biochem. 1989;180:195–204.
Palmisano G, Melo-Braga MN, Engholm-Keller K, Parker BL, Larsen MR. Chemical deamidation: a common pitfall in large-scale N-linked glycoproteomic mass spectrometry-based analyses. J Proteome Res. 2012;11:1949–57.
Karczewski KJ, Francioli LC, Tiao G, Cummings BB, Alfoldi J, Wang Q, et al. The mutational constraint spectrum quantified from variation in 141,456 humans. Nature. 2020;581:434–43.
Hernandez RD, Uricchio LH, Hartman K, Ye C, Dahl A, Zaitlen N. Ultrarare variants drive substantial cis heritability of human gene expression. Nat Genet. 2019;51:1349–55.
Roussel A, Spinelli S, Deret S, Navaza J, Aucouturier P, Cambillau C. The structure of an entire noncovalent immunoglobulin kappa light-chain dimer (Bence-Jones protein) reveals a weak and unusual constant domains association. Eur J Biochem. 1999;260:192–9.
Ohtsubo K, Marth JD. Glycosylation in cellular mechanisms of health and disease. Cell. 2006;126:855–67.
Helenius A, Aebi M. Intracellular functions of N-linked glycans. Science. 2001;291:2364–9.
Gudelj I, Lauc G, Pezer M. Immunoglobulin G glycosylation in aging and diseases. Cell Immunol. 2018;333:65–79.
Quast I, Keller CW, Maurer MA, Giddens JP, Tackenberg B, Wang LX, et al. Sialylation of IgG Fc domain impairs complement-dependent cytotoxicity. J Clin Investig. 2015;125:4160–70.
Arnold JN, Wormald MR, Sim RB, Rudd PM, Dwek RA. The impact of glycosylation on the biological function and structure of human immunoglobulins. Annu Rev Immunol. 2007;25:21–50.
Dunn-Walters D, Boursier L, Spencer J. Effect of somatic hypermutation on potential N-glycosylation sites in human immunoglobulin heavy chain variable regions. Mol Immunol. 2000;37:107–13.
Anumula KR. Quantitative glycan profiling of normal human plasma derived immunoglobulin and its fragments Fab and Fc. J Immunol Methods. 2012;382:167–76.
Sabouri Z, Schofield P, Horikawa K, Spierings E, Kipling D, Randall KL, et al. Redemption of autoantibodies on anergic B cells by variable-region glycosylation and mutation away from self-reactivity. Proc Natl Acad Sci USA. 2014;111:E2567–75.
Zhu D, McCarthy H, Ottensmeier CH, Johnson P, Hamblin TJ, Stevenson FK. Acquisition of potential N-glycosylation sites in the immunoglobulin variable region by somatic mutation is a distinctive feature of follicular lymphoma. Blood. 2002;99:2562–8.
Zhu D, Ottensmeier CH, Du MQ, McCarthy H, Stevenson FK. Incidence of potential glycosylation sites in immunoglobulin variable regions distinguishes between subsets of Burkitt’s lymphoma and mucosa-associated lymphoid tissue lymphoma. Br J Haematol. 2003;120:217–22.
Forconi F, Capello D, Berra E, Rossi D, Gloghini A, Cerri M, et al. Incidence of novel N-glycosylation sites in the B-cell receptor of lymphomas associated with immunodeficiency. Br J Haematol. 2004;124:604–9.
Zabalegui N, de Cerio AL, Inoges S, Rodriguez-Calvillo M, Perez-Calvo J, Hernandez M, et al. Acquired potential N-glycosylation sites within the tumor-specific immunoglobulin heavy chains of B-cell malignancies. Haematologica. 2004;89:541–6.
Coelho V, Krysov S, Ghaemmaghami AM, Emara M, Potter KN, Johnson P, et al. Glycosylation of surface Ig creates a functional bridge between human follicular lymphoma and microenvironmental lectins. Proc Natl Acad Sci USA. 2010;107:18587–92.
Linley A, Krysov S, Ponzoni M, Johnson PW, Packham G, Stevenson FK. Lectin binding to surface Ig variable regions provides a universal persistent activating signal for follicular lymphoma cells. Blood. 2015;126:1902–10.
Amin R, Mourcin F, Uhel F, Pangault C, Ruminy P, Dupre L, et al. DC-SIGN-expressing macrophages trigger activation of mannosylated IgM B-cell receptor in follicular lymphoma. Blood. 2015;126:1911–20.
Bellotti V, Mangione P, Merlini G. Review: immunoglobulin light chain amyloidosis—the archetype of structural and pathogenic variability. J Struct Biol. 2000;130:280–9.
Zhang C, Huang X, Li J. Light chain amyloidosis: Where are the light chains from and how they play their pathogenic role? Blood Rev. 2017;31:261–70.
Blancas-Mejia LM, Misra P, Dick CJ, Cooper SA, Redhage KR, Bergman MR, et al. Immunoglobulin light chain amyloid aggregation. Chem Commun. 2018;54:10664–74.
Radamaker L, Karimi-Farsijani S, Andreotti G, Baur J, Neumann M, Schreiner S, et al. Role of mutations and post-translational modifications in systemic AL amyloidosis studied by cryo-EM. Nat Commun. 2021;12:6434.
Murray DL, Puig N, Kristinsson S, Usmani SZ, Dispenzieri A, Bianchi G, et al. Mass spectrometry for the evaluation of monoclonal proteins in multiple myeloma and related disorders: an International Myeloma Working Group Mass Spectrometry Committee Report. Blood Cancer J. 2021;11:24.
Blancas-Mejia LM, Horn TJ, Marin-Argany M, Auton M, Tischer A, Ramirez-Alvarado M. Thermodynamic and fibril formation studies of full length immunoglobulin light chain AL-09 and its germline protein using scan rate dependent thermal unfolding. Biophys Chem. 2015;207:13–20.
Klimtchuk ES, Gursky O, Patel RS, Laporte KL, Connors LH, Skinner M, et al. The critical role of the constant region in thermal stability and aggregation of amyloidogenic immunoglobulin light chain. Biochemistry. 2010;49:9848–57.
Gonzalez-Andrade M, Becerril-Lujan B, Sanchez-Lopez R, Cecena-Alvarez H, Perez-Carreon JI, Ortiz E, et al. Mutational and genetic determinants of lambda6 light chain amyloidogenesis. FEBS J. 2013;280:6173–83.
Maritan M, Ambrosetti A, Oberti L, Barbiroli A, Diomede L, Romeo M, et al. Modulating the cardiotoxic behaviour of immunoglobulin light chain dimers through point mutations. Amyloid. 2019;26:105–6.
Rottenaicher GJ, Weber B, Ruhrnossl F, Kazman P, Absmeier RM, Hitzenberger M, et al. Molecular mechanism of amyloidogenic mutations in hypervariable regions of antibody light chains. J Biol Chem. 2021;296:100334.
Kazman P, Vielberg MT, Pulido Cendales MD, Hunziger L, Weber B, Hegenbart U, et al. Fatal amyloid formation in a patient’s antibody light chain is caused by a single point mutation. Elife. 2020;9:e52300.
Weber B, Hora M, Kazman P, Gobl C, Camilloni C, Reif B, et al. The antibody light-chain linker regulates domain orientation and amyloidogenicity. J Mol Biol. 2018;430:4925–40.
Morgan GJ, Kelly JW. The kinetic stability of a full-length antibody light chain dimer determines whether endoproteolysis can release amyloidogenic variable domains. J Mol Biol. 2016;428:4280–97.
Morgan GJ, Usher GA, Kelly JW. Incomplete refolding of antibody light chains to non-native, protease-sensitive conformations leads to aggregation: a mechanism of amyloidogenesis in patients? Biochemistry. 2017;56:6597–614.
Rennella E, Morgan GJ, Kelly JW, Kay LE. Role of domain interactions in the aggregation of full-length immunoglobulin light chains. Proc Natl Acad Sci USA. 2019;116:854–63.
Moriconi C, Ordonez A, Lupo G, Gooptu B, Irving JA, Noto R, et al. Interactions between N-linked glycosylation and polymerisation of neuroserpin within the endoplasmic reticulum. FEBS J. 2015;282:4565–79.
Visentin C, Broggini L, Sala BM, Russo R, Barbiroli A, Santambrogio C, et al. Glycosylation tunes neuroserpin physiological and pathological properties. Int J Mol Sci. 2020;21:3235.
Acknowledgements
We thank Andrea Patrignani and staff of the Functional Genomic Center Zurich for PacBio library preparation and sequencing. This work was supported by grants from the Amyloidosis Foundation (MNu), the Italian Ministry of Health (Ricerca Finalizzata, grant #GR-2018-12368387) (MNu), the Italian Ministry of Research and Education (PRIN 20207XLJB2) (SR, GP), the CARIPLO Foundation (grant #2018-0257) (MNu), Fondazione ARISLA (project TDP-43-STRUCT) (SR), Cancer Research UK [C355/A26819], FC AECC and AIRC under the Accelerator Award Program (BP, GM, GP, MNu). We would like to acknowledge the use of the Boston University AL-Base, supported by HL68705, in this work.
Author information
Authors and Affiliations
Contributions
Conceived, designed, and supervised the project: MNu. Provided financial support to the project: SR, BP, GMe, GP, MNu. Performed clinical evaluations, provided patients’ samples and/or collected clinical data: SM, BP, PM, CCS, PB, IG, FF, MB, AF, MNa, MTP, LA, GMe, GP, MNu. Maintained biorepository: AN, MG, PC, SCam, MAS, SCas. Curated clinical database: AN, PB, FF, MNu. Retrieved published sequences and performed in silico analyses: AN, MG, PC, MP, PR, MNu. Processed bone-marrow samples, prepared sequencing libraries and analyzed LC sequences: AN, MG, PC, MP, MNu. Performed PNGase F digestion and Western blotting: MG, PC, MP. Performed mass spectrometry analyses: GMa, SCam, FL. Performed genomic analyses and Sanger sequencing: AN, MG, PC, MP, SCas. Performed molecular modeling and structural analyses: VS, SR. Performed statistical analyses: PPO. Prepared figures: AN, PC, MP, VS, SR, MNu. Wrote the paper: AN, MNu. Read, edited and approved the paper: All authors.
Corresponding authors
Ethics declarations
Competing interests
PC, GP, and MNu are inventors on a patent application related to immunoglobulin sequencing.
Ethics approval and consent to participate
Clinical records and biological samples were from subjects referred to the Italian Amyloid Center or to the Department of Hematology, Fondazione IRCCS Policlinico San Matteo, Pavia, Italy and to the University Hospital of Navarra, Pamplona, Spain for a diagnostic workout in the suspicion of systemic AL amyloidosis or MM. Per the Declaration of Helsinki, all patients gave their written informed consent for the use of their clinical data and biological samples for research purposes, and this study was approved by the local Institutional Review Board.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Nevone, A., Girelli, M., Mangiacavalli, S. et al. An N-glycosylation hotspot in immunoglobulin κ light chains is associated with AL amyloidosis. Leukemia 36, 2076–2085 (2022). https://doi.org/10.1038/s41375-022-01599-w
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41375-022-01599-w