Abstract
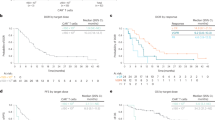
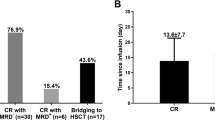
Down syndrome-associated acute lymphoblastic leukemia (DS-ALL) patients suffer risk of chemotherapy-associated toxicities and poor outcomes. We evaluated tisagenlecleucel in 16 patients with DS-ALL in two phase 2 trials (ELIANA [NCT02435849], ENSIGN [NCT02228096]) and a phase 3b, managed access protocol (B2001X [NCT03123939]). Patients were 5–22 years old, had a median of two prior lines of therapy (range, 1–4), and four (25%) had prior stem cell transplants. Fourteen of 16 patients (88%) achieved complete remission (CR) or CR with incomplete blood count recovery (CRi); 12 of 14 (86%) with CR/CRi were minimal residual disease-negative. With a median follow-up of 13.2 months (range, 0.5–49.3 months), six patients (43%) relapsed after CR (three, CD19-negative; three, unknown) between 80–721 days post-infusion. Ongoing remissions in nine patients ranged from 6–48 months. Any-grade and grade 3/4 AEs occurred in 16 and 14 patients, respectively; 44% experienced grade 3/4 cytokine release syndrome and 13% experienced grade 3/4 neurological events. Grade 3/4 prolonged cytopenias occurred in 44% of patients. No grade 3/4 infections were observed. Tisagenlecleucel expansion and long-term persistence were consistent with previous reports. Comparable to ALL patients without DS, tisagenlecleucel produced high remission rates, manageable side-effects, and promising long-term outcomes in pediatric/young adult patients with DS-ALL.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
Data availability
Novartis is committed to sharing with qualified external researchers access to patient-level data and supporting clinical documents from eligible studies. These requests are reviewed and approved by an independent review panel on the basis of scientific merit. All data provided are anonymized to respect the privacy of patients who have participated in the trial in line with applicable laws and regulations. The data availability of these trials is according to the criteria and process described on www.clinicalstudydatarequest.com.
References
Satge D, Seidel MG. The pattern of malignancies in Down syndrome and its potential context with the immune system. Front Immunol. 2018;9:3058.
Ross JA, Spector LG, Robison LL, Olshan AF. Epidemiology of leukemia in children with Down syndrome. Pediatr Blood Cancer. 2005;44:8–12.
Hasle H, Clemmensen IH, Mikkelsen M. Risks of leukaemia and solid tumours in individuals with Down’s syndrome. Lancet 2000;355:165–9.
Whitlock JA. Down syndrome and acute lymphoblastic leukaemia. Br J Haematol. 2006;135:595–602.
Peeters M, Poon A. Down syndrome and leukemia: unusual clinical aspects and unexpected methotrexate sensitivity. Eur J Pediatr. 1987;146:416–22.
Lee P, Bhansali R, Izraeli S, Hijiya N, Crispino JD. The biology, pathogenesis and clinical aspects of acute lymphoblastic leukemia in children with Down syndrome. Leukemia 2016;30:1816–23.
Meyr F, Escherich G, Mann G, Klingebiel T, Kulozik A, Rossig C, et al. Outcomes of treatment for relapsed acute lymphoblastic leukaemia in children with Down syndrome. Br J Haematol. 2013;162:98–106.
Hitzler JK, He W, Doyle J, Cairo M, Camitta BM, Chan KW, et al. Outcome of transplantation for acute lymphoblastic leukemia in children with Down syndrome. Pediatr Blood Cancer. 2014;61:1126–8.
Buitenkamp TD, Izraeli S, Zimmermann M, Forestier E, Heerema NA, van den Heuvel-Eibrink MM, et al. Acute lymphoblastic leukemia in children with Down syndrome: a retrospective analysis from the Ponte di Legno study group. Blood 2014;123:70–7.
Athale UH, Puligandla M, Stevenson KE, Asselin B, Clavell LA, Cole PD, et al. Outcome of children and adolescents with Down syndrome treated on Dana-Farber Cancer Institute Acute Lymphoblastic Leukemia Consortium protocols 00-001 and 05-001. Pediatr Blood Cancer. 2018;65:e27256.
32nd Annual Meeting and Pre-Conference Programs of the Society for Immunotherapy of Cancer (SITC 2017): Part One. J ImmunoTher Cancer. 2017;5.
Maude SL, Laetsch TW, Buechner J, Rives S, Boyer M, Bittencourt H, et al. Tisagenlecleucel in children and young adults with B-cell lymphoblastic leukemia. N. Engl J Med. 2018;378:439–48.
Maude SL, Grupp SA, Mody R, Driscoll T, Laetsch TW, Qayed M, et al. An updated analysis of tisagenlecleucel in pediatric/young adult patients with relapsed/refractory B-cell acute lymphoblastic leukemia (B-ALL) in a US multicenter clinical trial (ENSIGN). HemaSphere. 2018;2:PF174.
Krueger J, Bittencourt H, Rives S, Baruchel A, De Moerloose B, Peters C, et al. Tisagenlecleucel (Tisa) for relapsed/refractory (r/r) acute lymphoblastic leukemia (ALL): B2001X study focusing on prior exposure to blinatumomab (BLINA) and inotuzumab (INO). Presented at 2020 ASCO Virtual Meeting; May 29–31, 2020; 10518.
Laetsch TW, Maude SL, Grupp SA, Boyer MW, Harris AC, Qayed M, et al. Tisagenlecleucel (CTL019) therapy appears safe and effective in pediatric patients with Down syndrome and relapsed/refractory acute lymphoblastic leukemia. N Engl J Med. 2018;378:439–48.
Li A, DiNofia A, Rheingold SR, Aplenc R, Callahan C, Baniewicz D. CD19 chimeric antigen receptor T cell therapy in Down syndrome patients with B-cell acute lymphoblastic leukemia. Cell Ther Transplant Canada Annu Meet. 2018;61.
Maude SL, Grupp S, Mody R, Driscoll T, Laetsch TW, Qayed M, et al. An updated analysis of tisagenlecleucel in pediatric/young adult patients with relapsed/refractory B-cell acute lymphoblastic leukemia (B-ALL) in a US multicenter clinical trial (ENSIGN). Presented at: 23rd European Hematology Association Congress; June 14–17, 2018; Stockholm, Sweden. PF174.
Porter DL, Levine BL, Kalos M, Bagg A, June CH. Chimeric antigen receptor-modified T cells in chronic lymphoid leukemia. N Engl J Med. 2011;365:725–33.
Mueller KT, Maude SL, Porter DL, Frey N, Wood P, Han X, et al. Cellular kinetics of CTL019 in relapsed/refractory B-cell acute lymphoblastic leukemia and chronic lymphocytic leukemia. Blood 2017;130:2317–25.
Mueller KT, Waldron E, Grupp SA, Levine JE, Laetsch TW, Pulsipher MA, et al. Clinical pharmacology of tisagenlecleucel in B-cell acute lymphoblastic leukemia. Clin Cancer Res. 2018;24:6175–84.
Hau EM, Caccia JN, Kasteler R, Spycher B, Suter T, Ammann RA, et al. Cardiovascular disease after childhood acute lymphoblastic leukaemia: a cohort study. Swiss Med Wkly. 2019;149:w20012.
Lipshultz SE. Exposure to anthracyclines during childhood causes cardiac injury. Semin Oncol 2006;33:S8–14.
Benhaourech S, Drighil A, Hammiri AE. Congenital heart disease and Down syndrome: various aspects of a confirmed association. Cardiovasc J Afr. 2016;27:287–90.
Burstein DS, Maude S, Grupp S, Griffis H, Rossano J, Lin K. Cardiac profile of chimeric antigen receptor T cell therapy in children: a single-institution experience. Biol Blood Marrow Transpl. 2018;24:1590–5.
Lee DW, Santomasso BD, Locke FL, Ghobadi A, Turtle CJ, Brudno JN, et al. ASTCT consensus grading for cytokine release syndrome and neurologic toxicity associated with immune effector cells. Biol Blood Marrow Transpl. 2019;25:625–38.
Maus MV, Alexander S, Bishop MR, Brudno JN, Callahan C, Davila ML, et al. Society for Immunotherapy of Cancer (SITC) clinical practice guideline on immune effector cell-related adverse events. J Immunother Cancer. 2020;8:e001511.
Pasquini MC, Hu ZH, Curran K, Laetsch T, Locke F, Rouce R, et al. Real-world evidence of tisagenlecleucel for pediatric acute lymphoblastic leukemia and non-Hodgkin lymphoma. Blood Adv. 2020;4:5414–24.
Li AM, Rabin KR, Kairalla J, Wang C, Devidas M, Militano O, et al. Blinatumomab associated seizure risk in patients with Down syndrome and B-lymphoblastic leukemia: An interim report from Children’s Oncology Group (COG) study AALL1731. Presented at: 63rd American Society of Hematology Annual Meeting and Exposition; December 11–14, 2021; Atlanta, GA. Poster 2340.
Acknowledgements
Medical writing support was provided by Michael Hobert, PhD, CMPP (Healthcare Consultancy Group) and was funded by Novartis Pharmaceuticals Corporation. This study was sponsored by Novartis Pharmaceuticals Corporation.
Author information
Authors and Affiliations
Contributions
All authors have contributed to the acquisition, analysis, or interpretation of data for this article; contributed to drafts of the article; revised the manuscript critically for important intellectual content; approved the final version to be published; and agreed to be accountable for all aspects of the article.
Corresponding author
Ethics declarations
Competing interests
TWL has received personal fees and nonfinancial support from Novartis, Cellectis, Bayer, Loxo Oncology, Eli Lilly, Deciphera, Jumo Health, and Y-mAbs Therapeutics; and research funding to his institution from Novartis, Pfizer, and Bayer. SLM has received clinical trial support from Novartis; and has served as a consultant or on advisory boards or study steering committees for Novartis, Kite, and Wugen. AB has received travel reimbursement and meeting support from Neovii and Medac; advisory board honorarium, speaker fees, and travel support from Novartis; and advisory board and speaker fees from Amgen. SR has received clinical trial support: consulting, advisory boards, travel reimbursement, and meeting support from Novartis, Shire/Servier, Cellectis, JazzPharma, Celgene, Kite/Gilead, and Amgen; and has served on a study steering committee for Novartis. HB has received consulting fees from Novartis Oncology, and personal fees and nonfinancial support from Jazz (outside the submitted work). MWB has received honoraria from Novartis. JB has received personal fees, advisory board/steering committee honoraria, and nonfinancial support from Novartis (during the conduct of the study); and advisory board honoraria from Pfizer, Kite, and Janssen (outside the submitted work). BDM has participated in an advisory board for Novartis; has received nonfinancial support from Articulate Science (during the conduct of the study); and has received nonfinancial support from Jazz Pharmaceuticals (outside the submitted work). MQ has received honoraria from Medexus, Jazz Pharmaceuticals, and Mesoblast, and has served as a consultant for Novartis. CLP has served as a member on the Board of Directors or Advisory Committees for Novartis and Incyte. MAP has received grants from Miltenyi and Adaptive; personal fees from Novartis, Adaptive, Mesoblast, Medexus, and Equillium. HH has received clinical trials support from Novartis. RT is an employee of Novartis. SAG has received grants and personal fees from Novartis, Jazz Pharmaceuticals, and CRISPR/Vertex; personal fees from Adaptimmune, TCR2 Therapeutics, Eureka Therapeutics, Cellectis, Roche, and Juno Therapeutics; and grants from Servier and Kite; and has been issued a patent for toxicity management for anti-tumor activity of CARs managed by the University of Pennsylvania.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Laetsch, T.W., Maude, S.L., Balduzzi, A. et al. Tisagenlecleucel in pediatric and young adult patients with Down syndrome-associated relapsed/refractory acute lymphoblastic leukemia. Leukemia 36, 1508–1515 (2022). https://doi.org/10.1038/s41375-022-01550-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41375-022-01550-z
This article is cited by
-
Recent progress in pediatric lymphoblastic leukemia
International Journal of Hematology (2023)
-
Tisagenlecleucel
Reactions Weekly (2022)