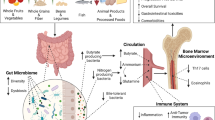
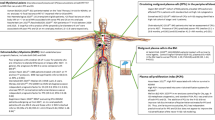
Abstract
Multiple myeloma (MM) remains an incurable plasma cell malignancy. Although little is known about the etiology of MM, several metabolic risk factors such as obesity, diabetes, poor nutrition, many of which are modifiable, have been linked to the pathogenesis of numerous neoplasms including MM. In this article, we provide a detailed summary of what is known about the impact of obesity on the pathogenesis of MM, its influence on outcomes in MM patients, and discuss potential mechanisms through which obesity is postulated to influence MM risk and prognosis. Along with advancements in treatment modalities to improve survival in MM patients, focused efforts are needed to prevent or intercept MM at its earliest stages. The consolidated findings presented in this review highlight the need for clinical trials to assess if lifestyle modifications can reduce the incidence and improve outcomes of MM in high-risk populations. Data generated from such studies can help formulate evidence-based lifestyle recommendations for the prevention and control of MM.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others
References
Kumar SK, Callander NS, Alsina M, Atanackovic D, Biermann JS, Castillo J, et al. NCCN guidelines insights: multiple myeloma, version 3.2018. J Natl Compr Canc Netw. 2018;16:11–20.
Hales CM, Carroll MD, Fryar CD, Ogden CL. Prevalence of obesity and severe obesity among adults: United States, 2017–2018. NCHS Data Brief. 2020:1–8.
Hales CM, Carroll MD, Fryar CD, Ogden CL. Prevalence of obesity among adults and youth: United States, 2015–2016. NCHS Data Brief. 2017:1–8.
Sung H, Siegel RL, Rosenberg PS, Jemal A. Emerging cancer trends among young adults in the USA: analysis of a population-based cancer registry. Lancet Public Health. 2019;4:e137–47.
Marinac CR, Birmann BM. Rising cancer incidence in younger adults: is obesity to blame? Lancet Public Health. 2019;4:e119–20.
Landgren O, Rajkumar SV, Pfeiffer RM, Kyle RA, Katzmann JA, Dispenzieri A, et al. Obesity is associated with an increased risk of monoclonal gammopathy of undetermined significance among black and white women. J Blood. 2010;116:1056–9.
Landgren O, Graubard BI, Katzmann JA, Kyle RA, Ahmadizadeh I, Clark R, et al. Racial disparities in the prevalence of monoclonal gammopathies: a population-based study of 12,482 persons from the National Health and Nutritional Examination Survey. Leukemia. 2014;28:1537–42.
Thordardottir M, Lindqvist EK, Lund SH, Costello R, Burton D, Korde N, et al. Obesity and risk of monoclonal gammopathy of undetermined significance and progression to multiple myeloma: a population-based study. Blood Adv. 2017;1:2186–92.
Chang SH, Luo S, Thomas TS, O'Brian KK, Colditz GA, Carlsson NP, et al. Obesity and the Transformation of Monoclonal Gammopathy of Undetermined Significance to Multiple Myeloma: A Population-Based Cohort Study. Journal of the National Cancer Institute. 2017;109.
Veld J, O’Donnell EK, Reagan MR, Yee AJ, Torriani M, Rosen CJ, et al. Abdominal adipose tissue in MGUS and multiple myeloma. Skelet Radiol. 2016;45:1277–83.
Samanic C, Gridley G, Chow WH, Lubin J, Hoover RN, Fraumeni JF Jr. Obesity and cancer risk among white and black United States veterans. Cancer Causes Control. 2004;15:35–43.
Blair CK, Cerhan JR, Folsom AR, Ross JA. Anthropometric characteristics and risk of multiple myeloma. Epidemiology. 2005;16:691–4.
Reeves GK, Pirie K, Beral V, Green J, Spencer E, Bull D. Cancer incidence and mortality in relation to body mass index in the Million Women Study: cohort study. BMJ. 2007;335:1134.
Engeland A, Tretli S, Hansen S, Bjørge T. Height and body mass index and risk of lymphohematopoietic malignancies in two million Norwegian men and women. Am J Epidemiol. 2007;165:44–52.
Söderberg KC, Kaprio J, Verkasalo PK, Pukkala E, Koskenvuo M, Lundqvist E, et al. Overweight, obesity and risk of haematological malignancies: a cohort study of Swedish and Finnish twins. Eur J Cancer. 2009;45:1232–8.
Brown LM, Gridley G, Pottern LM, Baris D, Swanso CA, Silverman DT, et al. Diet and nutrition as risk factors for multiple myeloma among blacks and whites in the United States. Cancer Causes Control. 2001;12:117–25.
Renehan AG, Tyson M, Egger M, Heller RF, Zwahlen M. Body-mass index and incidence of cancer: a systematic review and meta-analysis of prospective observational studies. Lancet. 2008;371:569–78.
Troy JD, Hartge P, Weissfeld JL, Oken MM, Colditz GA, Mechanic LE, et al. Associations between anthropometry, cigarette smoking, alcohol consumption, and non-Hodgkin lymphoma in the prostate, lung, colorectal, and ovarian cancer screening trial. Am J Epidemiol. 2010;171:1270–81.
Hofmann JN, Moore SC, Lim U, Park Y, Baris D, Hollenbeck AR, et al. Body mass index and physical activity at different ages and risk of multiple myeloma in the NIH-AARP diet and health study. Am J Epidemiol. 2013;177:776–86.
Birmann BM, Andreotti G, De Roos AJ, Camp NJ, Chiu BCH, Spinelli JJ, et al. Young adult and usual adult body mass index and multiple myeloma risk: a pooled analysis in the international multiple myeloma consortium (IMMC). Cancer Epidemiol Biomarkers Prev. 2017;26:876–85.
Marinac CR, Birmann BM, Lee IM, Rosner BA, Townsend MK, Giovannucci E, et al. Body mass index throughout adulthood, physical activity, and risk of multiple myeloma: a prospective analysis in three large cohorts. Br J Cancer. 2018;118:1013–9.
Marinac CR, Suppan CA, Giovannucci E, Song M, Kværner AS, Townsend MK, et al. Elucidating under-studied aspects of the link between obesity and multiple myeloma: weight pattern, body shape trajectory, and body fat distribution. JNCI Cancer Spectr. 2019;3:pkz044.
Samanic C, Chow WH, Gridley G, Jarvholm B, Fraumeni JF Jr. Relation of body mass index to cancer risk in 362,552 Swedish men. Cancer Causes Control. 2006;17:901–9.
Britton JA, Khan AE, Rohrmann S, Becker N, Linseisen J, Nieters A, et al. Anthropometric characteristics and non-Hodgkin’s lymphoma and multiple myeloma risk in the European Prospective Investigation into Cancer and Nutrition (EPIC). Haematologica. 2008;93:1666–77.
Fernberg P, Odenbro A, Bellocco R, Boffetta P, Pawitan Y, Zendehdel K, et al. Tobacco use, body mass index, and the risk of leukemia and multiple myeloma: a nationwide cohort study in Sweden. Cancer Res. 2007;67:5983–6.
Kanda J, Matsuo K, Inoue M, Iwasaki M, Sawada N, Shimazu T, et al. Association of anthropometric characteristics with the risk of malignant lymphoma and plasma cell myeloma in a Japanese population: a population-based cohort study. Cancer Epidemiol Biomarkers Prev. 2010;19:1623–31.
Lu Y, Sullivan-Halley J, Henderson KD, Ma H, Horn-Ross PL, Reynolds P, et al. Anthropometric characteristics and multiple myeloma risk. Epidemiology. 2010;21:272–3.
Pylypchuk RD, Schouten LJ, Goldbohm RA, Schouten HC, van den Brandt PA. Body mass index, height, and risk of lymphatic malignancies: a prospective cohort study. Am J Epidemiol. 2009;170:297–307.
Calle EE, Rodriguez C, Walker-Thurmond K, Thun MJ. Overweight, obesity, and mortality from cancer in a prospectively studied cohort of US adults. N Engl J Med. 2003;348:1625–38.
Khan MM, Mori M, Sakauchi F, Matsuo K, Ozasa K, Tamakoshi A. Risk factors for multiple myeloma: evidence from the Japan Collaborative Cohort (JACC) study. Asian Pac J Cancer Prev. 2006;7:575–81.
Teras LR, Kitahara CM, Birmann BM, Hartge PA, Wang SS, Robien K, et al. Body size and multiple myeloma mortality: a pooled analysis of 20 prospective studies. Br J Haematol. 2014;166:667–76.
Sonderman JS, Bethea TN, Kitahara CM, Patel AV, Harvey C, Knutsen SF, et al. Multiple myeloma mortality in relation to obesity among African Americans. J Natl Cancer Inst. 2016;108.
Beason TS, Chang SH, Sanfilippo KM, Luo S, Colditz GA, Vij R, et al. Influence of body mass index on survival in veterans with multiple myeloma. Oncologist. 2013;18:1074–9.
Williams A, Baruah D, Patel J, Szabo A, Chhabra S, Dhakal B, et al. Prevalence and significance of sarcopenia in multiple myeloma patients undergoing autologous hematopoietic cell transplantation. Bone marrow transplantation. 2021;56:225–31.
Vogl DT, Wang T, Pérez WS, Stadtmauer EA, Heitjan DF, Lazarus HM, et al. Effect of obesity on outcomes after autologous hematopoietic stem cell transplantation for multiple myeloma. Biol Blood Marrow Transplant. 2011;17:1765–74.
Ershler R, Fernandes LL, Kanapuru B, Gwise T, Kluetz PG, Theoret MR, et al. FDA analysis: Impact of BMI on efficacy outcomes in multiple myeloma trials. J Clin Oncol. 2020;38 15_suppl:8543.
Roy V, Swaika A, Kumar S, Mikhael J, Chanan-Khan A, Lacy M, et al. Influence of obesity on outcomes of patients with relapsed refractory multiple myeloma. Clin Lymphoma Myeloma Leuk. 2017;17:e139–40.
Caan BJ, Cespedes Feliciano EM, Kroenke CH. The importance of body composition in explaining the overweight paradox in cancer-counterpoint. Cancer Res. 2018;78:1906–12.
Nieman KM, Romero IL, Van Houten B, Lengyel E. Adipose tissue and adipocytes support tumorigenesis and metastasis. Biochim Biophys Acta. 2013;1831:1533–41.
Colotta F, Allavena P, Sica A, Garlanda C, Mantovani A. Cancer-related inflammation, the seventh hallmark of cancer: links to genetic instability. Carcinogenesis. 2009;30:1073–81.
Kidane D, Chae WJ, Czochor J, Eckert KA, Glazer PM, Bothwell AL, et al. Interplay between DNA repair and inflammation, and the link to cancer. Crit Rev Biochem Mol Biol. 2014;49:116–39.
Bullwinkle EM, Parker MD, Bonan NF, Falkenberg LG, Davison SP, DeCicco-Skinner KL. Adipocytes contribute to the growth and progression of multiple myeloma: unraveling obesity related differences in adipocyte signaling. Cancer Lett. 2016;380:114–21.
Hefetz-Sela S, Scherer PE. Adipocytes: impact on tumor growth and potential sites for therapeutic intervention. Pharmacol Thera. 2013;138:197–210.
Li Z, Liu H, He J, Wang Z, Yin Z, You G, et al. Acetyl-CoA synthetase 2: a critical linkage in obesity-induced tumorigenesis in myeloma. Cell Metab. 2021;33:78–93.e7.
Cawthorn WP, Scheller EL, Parlee SD, Pham HA, Learman BS, Redshaw CM, et al. Expansion of bone marrow adipose tissue during caloric restriction is associated with increased circulating glucocorticoids and not with hypoleptinemia. Endocrinology. 2016;157:508–21.
Caers J, Deleu S, Belaid Z, De Raeve H, Van Valckenborgh E, De Bruyne E, et al. Neighboring adipocytes participate in the bone marrow microenvironment of multiple myeloma cells. Leukemia. 2007;21:1580–4.
Rosen CJ, Ackert-Bicknell C, Rodriguez JP, Pino AM. Marrow fat and the bone microenvironment: developmental, functional, and pathological implications. Crit Rev Eukaryot Gene Expr. 2009;19:109–24.
Falank C, Fairfield H, Reagan MR. Signaling interplay between bone marrow adipose tissue and multiple myeloma cells. Front Endocrinol. 2016;7:67.
Pan SY, Johnson KC, Ugnat AM, Wen SW, Mao Y. Association of obesity and cancer risk in Canada. Am J Epidemiol. 2004;159:259–68.
Wallin A, Larsson SC. Body mass index and risk of multiple myeloma: a meta-analysis of prospective studies. Eur J Cancer. 2011;47:1606–15.
Liu Z, Xu J, He J, Liu H, Lin P, Wan X, et al. Mature adipocytes in bone marrow protect myeloma cells against chemotherapy through autophagy activation. Oncotarget. 2015;6:34329.
Rajkumar SV, Leong T, Roche PC, Fonseca R, Dispenzieri A, Lacy MQ, et al. Prognostic value of bone marrow angiogenesis in multiple myeloma. Clin Cancer Res. 2000;6:3111–6.
Roberts DL, Dive C, Renehan AG. Biological mechanisms linking obesity and cancer risk: new perspectives. Annu Rev Med. 2010;61:301–16.
Greenberg AS, Obin MS. Obesity and the role of adipose tissue in inflammation and metabolism. Am J Clin Nutr. 2006;83:461s–5s.
Dalamaga M, Karmaniolas K, Panagiotou A, Hsi A, Chamberland J, Dimas C, et al. Low circulating adiponectin and resistin, but not leptin, levels are associated with multiple myeloma risk: a case-control study. Cancer Causes Control. 2009;20:193–9.
Hofmann JN, Liao LM, Pollak MN, Wang Y, Pfeiffer RM, Baris D, et al. A prospective study of circulating adipokine levels and risk of multiple myeloma. Blood. 2012;120:4418–20.
Hofmann JN, Birmann BM, Teras LR, Pfeiffer RM, Wang Y, Albanes D, et al. Low levels of circulating adiponectin are associated with multiple myeloma risk in overweight and obese individuals. Cancer Res. 2016;76:1935–41.
Hofmann JN, Mailankody S, Korde N, Wang Y, Tageja N, Costello R, et al. Circulating adiponectin levels differ between patients with multiple myeloma and its precursor disease. Obesity. 2017;25:1317–20.
Peterson SJ, Drummond G, Kim DH, Li M, Kruger AL, Ikehara S, et al. L-4F treatment reduces adiposity, increases adiponectin levels, and improves insulin sensitivity in obese mice. J lipid Res. 2008;49:1658–69.
Fowler JA, Lwin ST, Drake MT, Edwards JR, Kyle RA, Mundy GR, et al. Host-derived adiponectin is tumor-suppressive and a novel therapeutic target for multiple myeloma and the associated bone disease. Blood. 2011;118:5872–82.
Medina EA, Oberheu K, Polusani SR, Ortega V, Velagaleti GV, Oyajobi BO. PKA/AMPK signaling in relation to adiponectin’s antiproliferative effect on multiple myeloma cells. Leukemia. 2014;28:2080–9.
Tanabe H, Fujii Y, Okada-Iwabu M, Iwabu M, Nakamura Y, Hosaka T, et al. Crystal structures of the human adiponectin receptors. Nature. 2015;520:312–6.
Ajuwon KM, Spurlock ME. Adiponectin inhibits LPS-induced NF-kappaB activation and IL-6 production and increases PPARgamma2 expression in adipocytes. Am J Physiol Regul Integr Comp Physiol. 2005;288:R1220–5.
Dalamaga M, Christodoulatos GS. Adiponectin as a biomarker linking obesity and adiposopathy to hematologic malignancies. Horm Mol Biol Clin Investig. 2015;23:5–20.
Steppan CM, Bailey ST, Bhat S, Brown EJ, Banerjee RR, Wright CM, et al. The hormone resistin links obesity to diabetes. Nature. 2001;409:307–12.
Yannakoulia M, Yiannakouris N, Blüher S, Matalas AL, Klimis-Zacas D, Mantzoros CS. Body fat mass and macronutrient intake in relation to circulating soluble leptin receptor, free leptin index, adiponectin, and resistin concentrations in healthy humans. J Clin Endocrinol Metab. 2003;88:1730–6.
Lee JH, Chan JL, Yiannakouris N, Kontogianni M, Estrada E, Seip R, et al. Circulating resistin levels are not associated with obesity or insulin resistance in humans and are not regulated by fasting or leptin administration: cross-sectional and interventional studies in normal, insulin-resistant, and diabetic subjects. J Clin Endocrinol Metab. 2003;88:4848–56.
Huang X, Yang Z. Resistin’s, obesity and insulin resistance: the continuing disconnect between rodents and humans. J Endocrinol Investig. 2016;39:607–15.
Savage DB, Sewter CP, Klenk ES, Segal DG, Vidal-Puig A, Considine RV, et al. Resistin/Fizz3 expression in relation to obesity and peroxisome proliferator-activated receptor-gamma action in humans. Diabetes. 2001;50:2199–202.
Tilg H, Moschen AR. Adipocytokines: mediators linking adipose tissue, inflammation and immunity. Nat Rev Immunol. 2006;6:772–83.
Lee S, Lee HC, Kwon YW, Lee SE, Cho Y, Kim J, et al. Adenylyl cyclase-associated protein 1 is a receptor for human resistin and mediates inflammatory actions of human monocytes. Cell Metab. 2014;19:484–97.
Santo L, Teras LR, Giles GG, Weinstein SJ, Albanes D, Wang Y, et al. Circulating resistin levels and risk of multiple myeloma in three prospective cohorts. Br J Cancer. 2017;117:1241–5.
Hosgood HD, Gunter MJ, Murphy N, Rohan TE, Strickler HD. The relation of obesity-related hormonal and cytokine levels with multiple myeloma and non-hodgkin lymphoma. Front Oncol. 2018;8:103.
Solary E, Guiguet M, Zeller V, Casasnovas RO, Caillot D, Chavanet P, et al. Radioimmunoassay for the measurement of serum IL-6 and its correlation with tumour cell mass parameters in multiple myeloma. Am J Hematol. 1992;39:163–71.
Lauta VM. A review of the cytokine network in multiple myeloma: diagnostic, prognostic, and therapeutic implications. Cancer. 2003;97:2440–52.
Kyrtsonis MC, Dedoussis G, Zervas C, Perifanis V, Baxevanis C, Stamatelou M, et al. Soluble interleukin-6 receptor (sIL-6R), a new prognostic factor in multiple myeloma. Br J Haematol. 1996;93:398–400.
Stasi R, Brunetti M, Parma A, Di Giulio C, Terzoli E, Pagano A. The prognostic value of soluble interleukin-6 receptor in patients with multiple myeloma. Cancer. 1998;82:1860–6.
Gadó K, Domján G, Hegyesi H, Falus A. Role of interleukin‐6 in the pathogenesis of multiple myeloma. Cell Biol Int. 2000;24:195–209.
Harmer D, Falank C, Reagan MR. Interleukin-6 interweaves the bone marrow microenvironment, bone loss, and multiple myeloma. Front Endocrinol. 2018;9:788.
Teoh G, Anderson KC. Interaction of tumor and host cells with adhesion and extracellular matrix molecules in the development of multiple myeloma. Hematol/Oncol Clin North Am. 1997;11:27–42.
Lust JA, Lacy MQ, Zeldenrust SR, Witzig TE, Moon-Tasson LL, Dinarello CA, et al. Reduction in C-reactive protein indicates successful targeting of the IL-1/IL-6 axis resulting in improved survival in early stage multiple myeloma. Am J Hematol. 2016;91:571–4.
Chakraborty R, Muchtar E, Kumar SK, Buadi FK, Dingli D, Dispenzieri A, et al. Elevated pre-transplant C-reactive protein identifies a high-risk subgroup in multiple myeloma patients undergoing delayed autologous stem cell transplantation. Bone Marrow Transplant. 2018;53:155–61.
Yang J, Wezeman M, Zhang X, Lin P, Wang M, Qian J, et al. Human C-reactive protein binds activating Fcgamma receptors and protects myeloma tumor cells from apoptosis. Cancer Cell. 2007;12:252–65.
Garrett IR, Durie BG, Nedwin GE, Gillespie A, Bringman T, Sabatini M, et al. Production of lymphotoxin, a bone-resorbing cytokine, by cultured human myeloma cells. N. Engl J Med. 1987;317:526–32.
Hideshima T, Chauhan D, Schlossman R, Richardson P, Anderson KC. The role of tumor necrosis factor alpha in the pathophysiology of human multiple myeloma: therapeutic applications. Oncogene. 2001;20:4519–27.
Fantuzzi G, Faggioni R. Leptin in the regulation of immunity, inflammation, and hematopoiesis. J Leukoc Biol. 2000;68:437–46.
Brennan AM, Mantzoros CS. Drug Insight: the role of leptin in human physiology and pathophysiology-emerging clinical applications. Nat Clin Pract Endocrinol Metab. 2006;2:318–27.
Baumann H, Morella KK, White DW, Dembski M, Bailon PS, Kim H, et al. The full-length leptin receptor has signaling capabilities of interleukin 6-type cytokine receptors. Proc Natl Acad Sci USA. 1996;93:8374–8.
Yang WH, Liu SC, Tsai CH, Fong YC, Wang SJ, Chang YS, et al. Leptin induces IL-6 expression through OBRl receptor signaling pathway in human synovial fibroblasts. PloS ONE. 2013;8:e75551.
Tang CH, Lu DY, Yang RS, Tsai HY, Kao MC, Fu WM, et al. Leptin-induced IL-6 production is mediated by leptin receptor, insulin receptor substrate-1, phosphatidylinositol 3-kinase, Akt, NF-kappaB, and p300 pathway in microglia. J Immunol. 2007;179:1292–302.
Reseland JE, Reppe S, Olstad OK, Hjorth-Hansen H, Brenne AT, Syversen U, et al. Abnormal adipokine levels and leptin-induced changes in gene expression profiles in multiple myeloma. Eur J Haematol. 2009;83:460–70.
Yu W, Cao DD, Li QB, Mei HL, Hu Y, Guo T. Adipocytes secreted leptin is a pro-tumor factor for survival of multiple myeloma under chemotherapy. Oncotarget. 2016;7:86075–86.
Colditz GA, Peterson LL. Obesity and cancer: evidence, impact, and future directions. Clin Chem. 2018;64:154–62.
Strickler HD, Wylie-Rosett J, Rohan T, Hoover DR, Smoller S, Burk RD, et al. The relation of type 2 diabetes and cancer. Diabetes Technol Ther. 2001;3:263–74.
Sprynski AC, Hose D, Kassambara A, Vincent L, Jourdan M, Rossi JF, et al. Insulin is a potent myeloma cell growth factor through insulin/IGF-1 hybrid receptor activation. Leukemia. 2010;24:1940–50.
Xu F, Gardner A, Tu Y, Michl P, Prager D, Lichtenstein A. Multiple myeloma cells are protected against dexamethasone-induced apoptosis by insulin-like growth factors. Br J Haematol. 1997;97:429–40.
Chang SH, Luo S, O’Brian KK, Thomas TS, Colditz GA, Carlsson NP, et al. Association between metformin use and progression of monoclonal gammopathy of undetermined significance to multiple myeloma in US veterans with diabetes mellitus: a population-based retrospective cohort study. Lancet Haematol. 2015;2:e30–6.
McTiernan A, Wu L, Chen C, Chlebowski R, Mossavar-Rahmani Y, Modugno F, et al. Relation of BMI and physical activity to sex hormones in postmenopausal women. Obesity. 2006;14:1662–77.
Peyrat JP, Bonneterre J, Beuscart R, Djiane J, Demaille A. Insulin-like growth factor 1 receptors in human breast cancer and their relation to estradiol and progesterone receptors. Cancer Res. 1988;48:6429–33.
Yu H, Shu XO, Li BD, Dai Q, Gao YT, Jin F, et al. Joint effect of insulin-like growth factors and sex steroids on breast cancer risk. Cancer Epidemiol Biomarkers Prev. 2003;12:1067–73.
Ellis MJ, Jenkins S, Hanfelt J, Redington ME, Taylor M, Leek R, et al. Insulin-like growth factors in human breast cancer. Breast Cancer Res Treat. 1998;52:175–84.
Grossmann M. Hypogonadism and male obesity: Focus on unresolved questions. Clin Endocrinol. 2018;89:11–21.
John S, Sharma N, Sborov DW, Williams N, Jones D, Benson DM, et al. Most multiple myeloma patients have low testosterone. Leuk Lymphoma. 2019;60:836–8.
Tchernof A, Labrie F. Dehydroepiandrosterone, obesity and cardiovascular disease risk: a review of human studies. Eur J Endocrinol. 2004;151:1–14.
Liu S, Ishikawa H, Li FJ, Ma Z, Otsuyama K, Asaoku H, et al. Dehydroepiandrosterone can inhibit the proliferation of myeloma cells and the interleukin-6 production of bone marrow mononuclear cells from patients with myeloma. Cancer Res. 2005;65:2269–76.
Acknowledgements
This research was funded in part through the NIH/NCI Cancer Center Support Grant (P30 CA008748) and supported by the American Society of Hematology Clinical Research Training Institute Award, TREC Training Workshop (R25 CA203650), International Myeloma Society and Paula and Rodger Riney Foundation CDA, Parker Institute of Cancer Immunotherapy CDA and NCI K12 CA184746 CDA (UAS). This study was funded in part by the National Institutes of Health (F32 CA220859, K22 CA251648), TREC Training Workshop (R25 CA203650) and the American Cancer Society (PF-17–231–01-CCE). This research was also supported by a Stand Up To Cancer Dream Team Research Grant (Grant Number: SU2C-AACR-DT28–18). Stand Up To Cancer is a program of the Entertainment Industry Foundation. Research grants are administered by the American Association for Cancer Research, the scientific partner of Stand Up To Cancer. Opinions, interpretations, conclusions and recommendations are those of the author(s) and are not necessarily endorsed by Stand Up To Cancer, the Entertainment Industry Foundation, or the American Association for Cancer Research (CRM).
Author information
Authors and Affiliations
Contributions
Concept and design: UAS and RP. Acquiring data: UAS, RP, and SMT. Drafting the paper: RP, SMT, CRM, and UAS. Critical revision of the paper: CRM and UAS. Approving the final version of the paper: RP, SMT, CRM, and UAS. Submission: RP and UAS. All authors agreed to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Corresponding author
Ethics declarations
Competing interests
UAS has received research funding from Celgene/Bristol Myers Squibb, Janssen to her institution and honorariums for continuing medical education activity from the Physicians Education Resource, all outside of the submitted work. RP, SMT, and CRM declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Parikh, R., Tariq, S.M., Marinac, C.R. et al. A comprehensive review of the impact of obesity on plasma cell disorders. Leukemia 36, 301–314 (2022). https://doi.org/10.1038/s41375-021-01443-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41375-021-01443-7
This article is cited by
-
Diet-induced obesity reduces bone marrow T and B cells and promotes tumor progression in a transplantable Vk*MYC model of multiple myeloma
Scientific Reports (2024)
-
Correlation Between Bariatric Surgery and the Risk of Multiple Myeloma: Results from an Evidence-Based Strategy
Obesity Surgery (2024)
-
Dietary and microbiome evidence in multiple myeloma and other plasma cell disorders
Leukemia (2023)
-
The roles of bone remodeling in normal hematopoiesis and age-related hematological malignancies
Bone Research (2023)
-
Extreme body mass index and survival in newly diagnosed multiple myeloma patients
Blood Cancer Journal (2023)