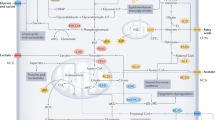
Abstract
Multiple Myeloma (MM) remains an incurable plasma cell neoplasm. Although little is known about the etiology of MM, several metabolic risk factors such as obesity, diabetes mellitus, diet, and the human intestinal microbiome have been linked to the pathogenesis of MM. In this article, we provide a detailed review of dietary and microbiome factors involved in the pathogenesis of MM and their impact on outcomes. Concurrent with treatment advancements that have improved survival in MM, focused efforts are needed to reduce the burden of MM as well as improve MM specific and overall outcomes once MM is diagnosed. The findings presented in this review will provide a comprehensive guide on the evidence available to date of the impact of dietary and other lifestyle interventions on the gut microbiome and on MM incidence, outcomes, and quality of life. Data generated from such studies can help formulate evidence-based guidelines for healthcare providers to counsel individuals at risk such as those with Monoclonal Gammopathy of Undetermined Significance (MGUS) and Smoldering Multiple Myeloma (SMM) as well as MM survivors with respect to their dietary habits.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others
References
Surveillance, Epidemiology, and End Results (SEER) Program (www.seer.cancer.gov) SEER*Stat Database: Incidence - SEER Research Data, 12 Registries, Nov 2021 Sub (1992-2019) - Linked To County Attributes - Time Dependent (1990-2019) Income/Rurality, 1969-2020 Counties, National Cancer Institute, DCCPS, Surveillance Research Program, released April 2022, based on the November 2021 submission.
Clinton SK, Giovannucci EL, Hursting SD. The world cancer research fund/American institute for cancer research third expert report on diet, nutrition, physical activity, and cancer: impact and future directions. J Nutr. 2020;150:663–71.
Malik MA, Sweeney NW, Jafri M, Derkach A, Chmielewski C, Adintori PA, et al. Nutrition perceptions, needs and practices among patients with plasma cell disorders. Blood Cancer J. 2022;12:70.
Almeida A, Mitchell AL, Boland M, Forster SC, Gloor GB, Tarkowska A, et al. A new genomic blueprint of the human gut microbiota. Nature. 2019;568:499–504.
Zhang B, Gu J, Liu J, Huang B, Li J. Fecal microbiota taxonomic shifts in Chinese multiple myeloma patients analyzed by quantitative polimerase chain reaction (QPCR) and 16S rRNA high-throughput sequencing. Med Sci Monit Int Med J Exp Clin Res. 2019;25:8269–80.
Vernocchi P, Del Chierico F, Putignani L. Gut microbiota metabolism and interaction with food components. Int J Mol Sci. 2020;21.
Honda K, Littman DR. The microbiota in adaptive immune homeostasis and disease. Nature. 2016;535:75–84.
Zhang FF, Cudhea F, Shan Z, Michaud DS, Imamura F, Eom H, et al. Preventable cancer burden associated with poor diet in the United States. JNCI Cancer Spectr. 2019;3:pkz034.
Brown LM, Gridley G, Pottern LM, Baris D, Swanso CA, Silverman DT, et al. Diet and nutrition as risk factors for multiple myeloma among blacks and whites in the United States. Cancer Causes Control CCC. 2001;12:117–25.
Hosgood HD 3rd, Baris D, Zahm SH, Zheng T, Cross AJ. Diet and risk of multiple myeloma in Connecticut women. Cancer Causes Control CCC. 2007;18:1065–76.
Key TJ, Appleby PN, Crowe FL, Bradbury KE, Schmidt JA, Travis RC. Cancer in British vegetarians: updated analyses of 4998 incident cancers in a cohort of 32,491 meat eaters, 8612 fish eaters, 18,298 vegetarians, and 2246 vegans. Am J Clin Nutr. 2014;100:378s–85s.
Lee DH, Fung TT, Tabung FK, Colditz GA, Ghobrial IM, Rosner BA, et al. Dietary pattern and risk of multiple myeloma in two large prospective US cohort studies. JNCI Cancer Spectr. 2019;3:pkz025.
Lee DH, Fung TT, Tabung FK, Marinac CR, Devore EE, Rosner BA, et al. Prediagnosis dietary pattern and survival in patients with multiple myeloma. Int J Cancer. 2020;147:1823–30.
Nagpal R, Shively CA, Register TC, Craft S, Yadav H. Gut microbiome-Mediterranean diet interactions in improving host health. F1000Res. 2019;8:699.
David LA, Maurice CF, Carmody RN, Gootenberg DB, Button JE, Wolfe BE, et al. Diet rapidly and reproducibly alters the human gut microbiome. Nature. 2014;505:559–63.
Peled JU, Gomes ALC, Devlin SM, Littmann ER, Taur Y, Sung AD, et al. Microbiota as predictor of mortality in allogeneic hematopoietic-cell transplantation. N Engl J Med. 2020;382:822–34.
Shah UA, Maclachlan KH, Derkach A, Salcedo M, Barnett K, Caple J, et al. Sustained minimal residual disease negativity in multiple myeloma is associated with stool butyrate and healthier plant-based diets. Clin Cancer Res. 2022;28:5149–55.
Thordardottir M, Lindqvist EK, Lund SH, Costello R, Burton D, Steingrimsdottir L, et al. Dietary intake is associated with risk of multiple myeloma and its precursor disease. PLoS One. 2018;13:e0206047.
Joseph JM, Tang L, Hillengass J, Moysich K, Landgren O, Usmani S, et al. Low intake of fruits and vegetables and high intake of processed meats and juices are associated with risk of mgus in the national health and nutrition examination survey (NHANES) database. Blood. 2022;140:12556–8.
Vlajinac HD, Pekmezović TD, Adanja BJ, Marinković JM, Kanazir MS, Suvajdzić ND, et al. Case-control study of multiple myeloma with special reference to diet as risk factor. Neoplasma. 2003;50:79–83.
Bang SJ, Kim G, Lim MY, Song EJ, Jung DH, Kum JS, et al. The influence of in vitro pectin fermentation on the human fecal microbiome. AMB Express. 2018;8:98.
Ramirez-Farias C, Slezak K, Fuller Z, Duncan A, Holtrop G, Louis P. Effect of inulin on the human gut microbiota: stimulation of Bifidobacterium adolescentis and Faecalibacterium prausnitzii. Br J Nutr. 2009;101:541–50.
Garcia-Mantrana I, Selma-Royo M, Alcantara C, Collado MC. Shifts on gut microbiota associated to mediterranean diet adherence and specific dietary intakes on general adult population. Front Microbiol. 2018;9:890.
Zhang Y, Kensler TW, Cho CG, Posner GH, Talalay P. Anticarcinogenic activities of sulforaphane and structurally related synthetic norbornyl isothiocyanates. Proc Natl Acad Sci USA. 1994;91:3147–50.
Bondonno NP, Dalgaard F, Kyrø C, Murray K, Bondonno CP, Lewis JR, et al. Flavonoid intake is associated with lower mortality in the Danish Diet Cancer and Health Cohort. Nat Commun. 2019;10:3651.
Kopustinskiene DM, Jakstas V, Savickas A, Bernatoniene J. Flavonoids as anticancer agents. Nutrients. 2020;12.
Block G. Vitamin C and cancer prevention: the epidemiologic evidence. Am J Clin Nutr. 1991;53:270s–82s.
Chatenoud L, Tavani A, La Vecchia C, Jacobs DR Jr., Negri E, Levi F, et al. Whole grain food intake and cancer risk. Int J Cancer. 1998;77:24–8.
La Vecchia C, Chatenoud L, Negri E, Franceschi S. Session: whole cereal grains, fibre and human cancer wholegrain cereals and cancer in Italy. Proc Nutr Soc. 2003;62:45–9.
Parikh R, Tariq SM, Marinac CR, Shah UA. A comprehensive review of the impact of obesity on plasma cell disorders. Leukemia. 2022;36:301–14.
Seal CJ, Courtin CM, Venema K, de Vries J. Health benefits of whole grain: effects on dietary carbohydrate quality, the gut microbiome, and consequences of processing. Compr Rev Food Sci Food Saf. 2021;20:2742–68.
Jefferson A, Adolphus K. The effects of intact cereal grain fibers, including wheat bran on the gut microbiota composition of healthy adults: a systematic review. Front Nutr. 2019;6:33.
Vitaglione P, Mennella I, Ferracane R, Rivellese AA, Giacco R, Ercolini D, et al. Whole-grain wheat consumption reduces inflammation in a randomized controlled trial on overweight and obese subjects with unhealthy dietary and lifestyle behaviors: role of polyphenols bound to cereal dietary fiber. Am J Clin Nutr. 2015;101:251–61.
Neacsu M, McMonagle J, Fletcher RJ, Hulshof T, Duncan SH, Scobbie L, et al. Availability and dose response of phytophenols from a wheat bran rich cereal product in healthy human volunteers. Mol Nutr Food Res. 2017;61.
Fritschi L, Ambrosini GL, Kliewer EV, Johnson KC. Dietary fish intake and risk of leukaemia, multiple myeloma, and non-Hodgkin lymphoma. Cancer epidemiology, biomarkers & prevention: a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive. Oncology. 2004;13:532–7.
Fernandez E, Chatenoud L, La Vecchia C, Negri E, Franceschi S. Fish consumption and cancer risk. Am J Clin Nutr. 1999;70:85–90.
Wang YZ, Wu QJ, Zhu J, Wu L. Fish consumption and risk of myeloma: a meta-analysis of epidemiological studies. Cancer Causes Control CCC. 2015;26:1307–14.
Landrigan PJ, Stegeman JJ, Fleming LE, Allemand D, Anderson DM, Backer LC, et al. Human health and ocean pollution. Ann Glob Health. 2020;86:151.
Parolini C. Effects of fish n-3 PUFAs on intestinal microbiota and immune system. Mar Drugs. 2019;17.
Tavani A, La Vecchia C, Gallus S, Lagiou P, Trichopoulos D, Levi F, et al. Red meat intake and cancer risk: a study in Italy. Int J Cancer. 2000;86:425–8.
Abu-Ghazaleh N, Chua WJ, Gopalan V. Intestinal microbiota and its association with colon cancer and red/processed meat consumption. J Gastroenterol Hepatol. 2021;36:75–88.
Gurjao C, Zhong R, Haruki K, Li YY, Spurr LF, Lee-Six H, et al. Discovery and features of an alkylating signature in colorectal cancer. Cancer Discov. 2021;11:2446–55.
Allen NE, Appleby PN, Davey GK, Kaaks R, Rinaldi S, Key TJ. The associations of diet with serum insulin-like growth factor I and its main binding proteins in 292 women meat-eaters, vegetarians, and vegans. Cancer Epidemiol, Biomarkers Prev. 2002;11:1441–8.
Knuppel A, Fensom GK, Watts EL, Gunter MJ, Murphy N, Papier K, et al. Circulating insulin-like growth factor-i concentrations and risk of 30 cancers: prospective analyses in UK Biobank. Cancer Res. 2020;80:4014–21.
Golombick T, Diamond TH, Manoharan A, Ramakrishna R. Monoclonal gammopathy of undetermined significance, smoldering multiple myeloma, and curcumin: a randomized, double-blind placebo-controlled cross-over 4g study and an open-label 8g extension study. Am J Hematol. 2012;87:455–60.
Golombick T, Diamond TH, Manoharan A, Ramakrishna R. Long-term follow-up of curcumin treated MGUS/SMM patients–an updated single centre experienceJ. Hematol Med Oncol. 2017;2.
Golombick T, Diamond TH, Manoharan A, Ramakrishna R. Long term use of curcumin in two smoldering multiple myeloma patients. J Hematol Malig. 2013;3:18–32.
Bharti AC, Donato N, Singh S, Aggarwal BB. Curcumin (diferuloylmethane) down-regulates the constitutive activation of nuclear factor-kappa B and IkappaBalpha kinase in human multiple myeloma cells, leading to suppression of proliferation and induction of apoptosis. Blood. 2003;101:1053–62.
Scazzocchio B, Minghetti L, D’Archivio M. Interaction between gut microbiota and curcumin: a new key of understanding for the health effects of curcumin. Nutrients. 2020;12.
Peterson CT, Vaughn AR, Sharma V, Chopra D, Mills PJ, Peterson SN, et al. Effects of turmeric and curcumin dietary supplementation on human gut microbiota: a double-blind, randomized, placebo-controlled pilot study. J Evid Based Integr Med. 2018;23:2515690X18790725.
Castro F, Sweeney NW, Derkach A, Traore K, Anuraj A, Guttentag L, et al. Microbial changes in response to a plant-based diet and/or supplements in SMM patients: a national multi-arm randomized prospective telehealth study via healthtree: the nutrition prevention (NUTRIVENTION-2) study. Blood. 2022;140:13079–81.
Shah UA, Castro F, Anuraj A, Schach E, Derkach A, Joseph NS, et al. A randomized placebo controlled study of a plant-based dietary versus supplement versus placebo intervention in patients with monoclonal gammopathy of undetermined significance (MGUS) and smoldering multiple myeloma (SMM) - the nutrition prevention (NUTRIVENTION-3) study. Blood. 2022;140:5052–5.
Burwick N. Vitamin D and plasma cell dyscrasias: reviewing the significance. Ann Hematol. 2017;96:1271–7.
Park WH, Seol JG, Kim ES, Hyun JM, Jung CW, Lee CC, et al. Induction of apoptosis by vitamin D3 analogue EB1089 in NCI-H929 myeloma cells via activation of caspase 3 and p38 MAP kinase. Br J Haematol. 2000;109:576–83.
Park WH, Seol JG, Kim ES, Jung CW, Lee CC, Binderup L, et al. Cell cycle arrest induced by the vitamin D(3) analog EB1089 in NCI-H929 myeloma cells is associated with induction of the cyclin-dependent kinase inhibitor p27. Exp Cell Res. 2000;254:279–86.
Badros A, Goloubeva O, Terpos E, Milliron T, Baer MR, Streeten E. Prevalence and significance of vitamin D deficiency in multiple myeloma patients. Br J Haematol. 2008;142:492–4.
Ng AC, Kumar SK, Rajkumar SV, Drake MT. Impact of vitamin D deficiency on the clinical presentation and prognosis of patients with newly diagnosed multiple myeloma. Am J Hematol. 2009;84:397–400.
Wang J, Udd KA, Vidisheva A, Swift RA, Spektor TM, Bravin E, et al. Low serum vitamin D occurs commonly among multiple myeloma patients treated with bortezomib and/or thalidomide and is associated with severe neuropathy. Support Care Cancer. 2016;24:3105–10.
Diamond T, Golombick T, Manoharan A. Vitamin D status may effect the skeletal complications of multiple myeloma. Am J Hematol. 2010;85:302–3.
Yellapragada SV, Fillmore NR, Frolov A, Zhou Y, Dev P, Yameen H, et al. Vitamin D deficiency predicts for poor overall survival in white but not African American patients with multiple myeloma. Blood Adv. 2020;4:1643–6.
Mohr SB, Gorham ED, Garland CF, Grant WB, Garland FC, Cuomo RE. Are low ultraviolet B and vitamin D associated with higher incidence of multiple myeloma? J Steroid Biochem Mol Biol. 2015;148:245–52.
Lauter B, Schmidt-Wolf IG. Prevalence, supplementation, and impact of vitamin D deficiency in multiple myeloma patients. Cancer Invest. 2015;33:505–9.
Eicher F, Mansouri Taleghani B, Schild C, Bacher U, Pabst T. Reduced survival after autologous stem cell transplantation in myeloma and lymphoma patients with low vitamin D serum levels. Hematol Oncol. 2020;38:523–30.
Nath K, Ganeshalingam V, Ewart B, Heyer E, Watt K, Birchley A, et al. A retrospective analysis of the prevalence and clinical outcomes of vitamin D deficiency in myeloma patients in tropical Australia. Support Care Cancer. 2020;28:1249–54.
Oortgiesen BE, Kroes JA, Scholtens P, Hoogland J, Dannenberg-de Keijzer P, Siemes C, et al. High prevalence of peripheral neuropathy in multiple myeloma patients and the impact of vitamin D levels, a cross-sectional study. Support Care Cancer. 2022;30:271–8.
Naderpoor N, Mousa A, Fernanda Gomez Arango L, Barrett HL, Dekker Nitert M, de Courten B. Effect of vitamin D supplementation on faecal microbiota: a randomised clinical trial. Nutrients. 2019;11.
Block G. Epidemiologic evidence regarding vitamin C and cancer. Am J Clin Nutr. 1991;54:1310s–4s.
Zou W, Yue P, Lin N, He M, Zhou Z, Lonial S, et al. Vitamin C inactivates the proteasome inhibitor PS-341 in human cancer cells. Clin Cancer Res Off J Am Assoc Cancer Res. 2006;12:273–80.
Perrone G, Hideshima T, Ikeda H, Okawa Y, Calabrese E, Gorgun G, et al. Ascorbic acid inhibits antitumor activity of bortezomib in vivo. Leukemia. 2009;23:1679–86.
Kumar GS, Das UN. Free radical-dependent suppression of growth of mouse myeloma cells by alpha-linolenic and eicosapentaenoic acids in vitro. Cancer Lett. 1995;92:27–38.
Mortaz E, Moloudizargari M, Khosravi A, Asghari MH, Movassaghi M, Varahram M, et al. EPA and DHA have selective toxicity for PBMCs from multiple myeloma patients in a partly caspase-dependent manner. Clin Nutr. 2020;39:2137–43.
Chen J, Garssen J, Redegeld F. The efficacy of bortezomib in human multiple myeloma cells is enhanced by combination with omega-3 fatty acids DHA and EPA: Timing is essential. Clin Nutr. 2021;40:1942–53.
Dai X, Li M, Geng F. Omega-3 polyunsaturated fatty acids eicosapentaenoic acid and docosahexaenoic acid enhance dexamethasone sensitivity in multiple myeloma cells by the p53/miR-34a/Bcl-2 Axis. Biochem (Mosc). 2017;82:826–33.
Maschio M, Zarabla A, Maialetti A, Marchesi F, Giannarelli D, Gumenyuk S, et al. The effect of docosahexaenoic acid and α-Lipoic acid as prevention of bortezomib-related neurotoxicity in patients with multiple myeloma. Integr Cancer Ther. 2019;18:1534735419888584.
Costantini L, Molinari R, Farinon B, Merendino N. Impact of Omega-3 fatty acids on the gut microbiota. Int J Mol Sci. 2017;18.
Abdi J, Garssen J, Faber J, Redegeld FA. Omega-3 fatty acids, EPA and DHA induce apoptosis and enhance drug sensitivity in multiple myeloma cells but not in normal peripheral mononuclear cells. J Nutr Biochem. 2014;25:1254–62.
D’Eliseo D, Di Renzo L, Santoni A, Velotti F. Docosahexaenoic acid (DHA) promotes immunogenic apoptosis in human multiple myeloma cells, induces autophagy and inhibits STAT3 in both tumor and dendritic cells. Genes Cancer. 2017;8:426–37.
Ciernikova S, Mego M, Semanova M, Wachsmannova L, Adamcikova Z, Stevurkova V, et al. Probiotic survey in cancer patients treated in the outpatient department in a comprehensive cancer center. Integr Cancer Ther. 2017;16:188–95.
Valdes AM, Walter J, Segal E, Spector TD. Role of the gut microbiota in nutrition and health. BMJ. 2018;361:k2179.
Boesmans L, Valles-Colomer M, Wang J, Eeckhaut V, Falony G, Ducatelle R, et al. Butyrate producers as potential next-generation probiotics: safety assessment of the administration of butyricicoccus pullicaecorum to healthy volunteers. mSystems. 2018;3.
Dizman N, Meza L, Bergerot P, Alcantara M, Dorff T, Lyou Y, et al. Nivolumab plus ipilimumab with or without live bacterial supplementation in metastatic renal cell carcinoma: a randomized phase 1 trial. Nat Med. 2022;28:704–12.
Spencer CN, McQuade JL, Gopalakrishnan V, McCulloch JA, Vetizou M, Cogdill AP, et al. Dietary fiber and probiotics influence the gut microbiome and melanoma immunotherapy response. Science. 2021;374:1632–40.
Badgeley A, Anwar H, Modi K, Murphy P, Lakshmikuttyamma A. Effect of probiotics and gut microbiota on anti-cancer drugs: Mechanistic perspectives. Biochim Biophys Acta Rev Cancer. 2021;1875:188494.
Washburn RL, Sandberg D, Gazdik, Stofer MA. Supplementation of a single species probiotic does not affect diversity and composition of the healthy adult gastrointestinal microbiome. Hum Nutr Metab. 2022;28:200148.
Yelin I, Flett KB, Merakou C, Mehrotra P, Stam J, Snesrud E, et al. Genomic and epidemiological evidence of bacterial transmission from probiotic capsule to blood in ICU patients. Nat Med. 2019;25:1728–32.
Suez J, Zmora N, Zilberman-Schapira G, Mor U, Dori-Bachash M, Bashiardes S, et al. Post-antibiotic gut mucosal microbiome reconstitution is impaired by probiotics and improved by autologous FMT. Cell. 2018;174:1406–23.e16.
Brevi A, Cogrossi LL, Lorenzoni M, Mattorre B, Bellone M. The insider: impact of the gut microbiota on cancer immunity and response to therapies in multiple myeloma. Front Immunol. 2022;13:845422.
Wastyk HC, Fragiadakis GK, Perelman D, Dahan D, Merrill BD, Yu FB, et al. Gut-microbiota-targeted diets modulate human immune status. Cell. 2021;184:4137–53.e14.
Psaltopoulou T, Sergentanis TN, Sergentanis IN, Karadimitris A, Terpos E, Dimopoulos MA. Alcohol intake, alcoholic beverage type and multiple myeloma risk: a meta-analysis of 26 observational studies. Leuk Lymphoma. 2015;56:1484–501.
Santo L, Liao LM, Andreotti G, Purdue MP, Hofmann JN. Alcohol consumption and risk of multiple myeloma in the NIH-AARP Diet and Health Study. Int J Cancer. 2019;144:43–8.
Díaz LE, Montero A, González-Gross M, Vallejo AI, Romeo J, Marcos A. Influence of alcohol consumption on immunological status: a review. Eur J Clin Nutr. 2002;56:S50–3.
Boissy P, Andersen TL, Abdallah BM, Kassem M, Plesner T, Delaissé JM. Resveratrol inhibits myeloma cell growth, prevents osteoclast formation, and promotes osteoblast differentiation. Cancer Res. 2005;65:9943–52.
Alrafas HR, Busbee PB, Nagarkatti M, Nagarkatti PS. Resveratrol modulates the gut microbiota to prevent murine colitis development through induction of Tregs and suppression of Th17 cells. J Leukoc Biol. 2019;106:467–80.
Adami J, Nyrén O, Bergström R, Ekbom A, Engholm G, Englund A, et al. Smoking and the risk of leukemia, lymphoma, and multiple myeloma (Sweden). Cancer Causes Control. 1998;9:49–56.
Andreotti G, Birmann BM, Cozen W, De Roos AJ, Chiu BC, Costas L, et al. A pooled analysis of cigarette smoking and risk of multiple myeloma from the international multiple myeloma consortium. Cancer Epidemiol Biomarkers Prev. 2015;24:631–4.
Engen PA, Green SJ, Voigt RM, Forsyth CB, Keshavarzian A. The Gastrointestinal microbiome: alcohol effects on the composition of intestinal microbiota. Alcohol Res. 2015;37:223–36.
Savin Z, Kivity S, Yonath H, Yehuda S. Smoking and the intestinal microbiome. Arch Microbiol. 2018;200:677–84.
Singh RK, Chang HW, Yan D, Lee KM, Ucmak D, Wong K, et al. Influence of diet on the gut microbiome and implications for human health. J Transl Med. 2017;15:73.
Shah UA, Derkach A, Castro F, Anuraj A, Blaslov J, Tran L, et al. A pilot plant based dietary intervention in MGUS and SMM patients with elevated BMI is feasible and associated with improvements in metabolic and microbiome biomarkers of progression. Blood. 2022;140:5066–9.
Antoine Pepeljugoski C, Morgan G, Braunstein M. Analysis of intestinal microbiome in multiple myeloma reveals progressive dysbiosis compared to MGUS and healthy individuals. Blood. 2019;134:3076.
Jian X, Zhu Y, Ouyang J, Wang Y, Lei Q, Xia J, et al. Alterations of gut microbiome accelerate multiple myeloma progression by increasing the relative abundances of nitrogen-recycling bacteria. Microbiome. 2020;8:74.
Calcinotto A, Brevi A, Chesi M, Ferrarese R, Garcia Perez L, Grioni M, et al. Microbiota-driven interleukin-17-producing cells and eosinophils synergize to accelerate multiple myeloma progression. Nat Commun. 2018;9:4832.
Bellone M, Brevi A, Huber S. Microbiota-propelled T helper 17 cells in inflammatory diseases and cancer. Microbiol Mol Biol Rev. 2020;84.
Johnson MO, Wolf MM, Madden MZ, Andrejeva G, Sugiura A, Contreras DC, et al. Distinct regulation of Th17 and Th1 cell differentiation by glutaminase-dependent metabolism. Cell. 2018;175:1780–95.e19.
Khan N, Lindner S, Gomes ALC, Devlin SM, Shah GL, Sung AD, et al. Fecal microbiota diversity disruption and clinical outcomes after auto-HCT: a multicenter observational study. Blood. 2021;137:1527–37.
El Jurdi N, Filali-Mouhim A, Salem I, Retuerto M, Dambrosio NM, Baer L, et al. Gastrointestinal microbiome and mycobiome changes during autologous transplantation for multiple myeloma: results of a prospective pilot study. Biol Blood Marrow Transplant. 2019;25:1511–9.
Laheij A, Raber-Durlacher JE, Koppelmans RGA, Huysmans M, Potting C, van Leeuwen SJM, et al. Microbial changes in relation to oral mucositis in autologous hematopoietic stem cell transplantation recipients. Sci Rep. 2019;9:16929.
Peled JU, Devlin SM, Staffas A, Lumish M, Khanin R, Littmann ER, et al. Intestinal microbiota and relapse after hematopoietic-cell transplantation. J Clin Oncol Off J Am Soc Clin Oncol. 2017;35:1650–9.
D’Angelo C, Sudakaran S, Asimakopoulos F, Hematti P, El-Gamal D, Safdar N, et al. Perturbation of the gut microbiome and association with outcomes following autologous stem cell transplantation in patients with multiple myeloma. Leuk Lymphoma. 2023;64:87–97.
McKenna M, Feinman R, Ahn J, Wang S, Gourna Paleoudis E, Vesole DH, et al. Severe infections and antibiotic use negatively impact progression free and overall survival of multiple myeloma patients undergoing autologous stem cell transplantation. Blood. 2019;134:5510.
Alkharabsheh O, Sidiqi MH, Aljama MA, Gertz MA, Frankel AE. The human microbiota in multiple myeloma and proteasome inhibitors. Acta Haematol. 2020;143:118–23.
Pianko MJ, Devlin SM, Littmann ER, Chansakul A, Mastey D, Salcedo M, et al. Minimal residual disease negativity in multiple myeloma is associated with intestinal microbiota composition. Blood Adv. 2019;3:2040–4.
McDonald D, Hyde E, Debelius JW, Morton JT, Gonzalez A, Ackermann G, et al. American gut: an open platform for citizen science microbiome research. mSystems. 2018;3.
Asnicar F, Berry SE, Valdes AM, Nguyen LH, Piccinno G, Drew DA, et al. Microbiome connections with host metabolism and habitual diet from 1,098 deeply phenotyped individuals. Nat Med. 2021;27:321–32.
Encarnação JC, Abrantes AM, Pires AS, Botelho MF. Revisit dietary fiber on colorectal cancer: butyrate and its role on prevention and treatment. Cancer Metastasis Rev. 2015;34:465–78.
Yao R, Han D, Sun X, Fu C, Wu Q, Yao Y, et al. Histone deacetylase inhibitor NaBut suppresses cell proliferation and induces apoptosis by targeting p21 in multiple myeloma. Am J Transl Res. 2017;9:4994–5002.
Li JY, Yu M, Pal S, Tyagi AM, Dar H, Adams J, et al. Parathyroid hormone-dependent bone formation requires butyrate production by intestinal microbiota. J Clin Invest. 2020;130:1767–81.
Tyagi AM, Yu M, Darby TM, Vaccaro C, Li JY, Owens JA, et al. The microbial metabolite butyrate stimulates bone formation via T regulatory cell-mediated regulation of WNT10B expression. Immunity. 2018;49:1116–31.e7.
Arpaia N, Campbell C, Fan X, Dikiy S, van der Veeken J, deRoos P, et al. Metabolites produced by commensal bacteria promote peripheral regulatory T-cell generation. Nature. 2013;504:451–5.
Bachem A, Makhlouf C, Binger KJ, de Souza DP, Tull D, Hochheiser K, et al. Microbiota-derived short-chain fatty acids promote the memory potential of antigen-activated CD8(+) T cells. Immunity. 2019;51:285–97.e5.
Vernieri C, Fuca G, Ligorio F, Huber V, Vingiani A, Iannelli F, et al. Fasting-mimicking diet is safe and reshapes metabolism and antitumor immunity in patients with cancer. Cancer Discov. 2022;12:90–107.
Acknowledgements
UAS received research support from the American Society of Hematology Clinical Research Training Institute, NIH/NCI Cancer Center Support Grant P30CA008748, Parker Institute for Cancer Immunotherapy, International Myeloma Society, Paula and Rodger Riney Foundation, TREC Training Workshop R25CA203650 (PI: Melinda Irwin), NCI MSK Paul Calabresi Career Development Award for Clinical Oncology K12 CA184746, HealthTree Foundation, and the Allen Foundation Inc. MB has received funding from Associazione Italiana per la Ricerca sul Cancro (AIRC) under IG2018- ID. 21808. MB was also supported by a grant from the Leukemia & Lymphoma Society (# 6618-21). AML received research support from NIH/NCI Cancer Center Support Grant P30CA008748, NCI 1R01CA249981–01, Sawiris Family Fund, and Paula and Rodger Riney Foundation.
Author information
Authors and Affiliations
Contributions
Concept and design: UAS, RP, AML. Acquiring data: UAS, RP, FC. Drafting the manuscript: UAS, RP, FC. Critical revision of the manuscript: UAS, RP, MB, AML. Approving the final version of the paper: UAS, RP, FC, MB, and AML. Submission: UAS and RP. UAS and RP contributed equally to this work. All authors agreed to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Corresponding author
Ethics declarations
Competing interests
UAS reports grants from NIH/NCI Cancer Center Support Grant P30CA008748, MSK Paul Calabresi Career Development Award for Clinical Oncology K12CA184746, Paula and Rodger Riney Foundation, Allen Foundation Inc, Parker Institute for Cancer Immunotherapy at MSK, HealthTree Foundation, and International Myeloma Society as well as non-financial support from American Society of Hematology Clinical Research Training Institute, TREC Training Workshop R25CA203650 (PI: Melinda Irwin). UAS also reports research funding support from Celgene/BMS, Janssen, Plantable, Sabinsa pharmaceuticals, VeggieDoctor and M and M labs to the institution, non-financial research support; personal fees from ACCC, MashUp MD, Janssen Biotech, Sanofi, BMS, MJH LifeSciences, Intellisphere, Phillips Gilmore Oncology Communications, and RedMedEd outside the submitted work. MB has received honoraria from Bristol Meyers Squibb; Co-owner of the patent # EP18209623.0 - Strategies to improve colonization and expression of Prevotella melaninogenica in the gut of patients affected by IL-17-mediated diseases. AML reports grants from Bristol Myers Squibb and Genentech; grants, personal fees, and non-financial support from Pfizer; and grants and personal fees from Janssen outside the submitted work. AML has a patent for US20150037346A1 licensed and with royalties paid from Serametrix, Inc. No disclosures were reported by the other authors.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Shah, U.A., Parikh, R., Castro, F. et al. Dietary and microbiome evidence in multiple myeloma and other plasma cell disorders. Leukemia 37, 964–980 (2023). https://doi.org/10.1038/s41375-023-01874-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41375-023-01874-4