Abstract
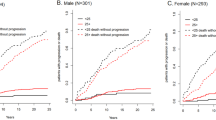
Our group previously demonstrated that M-protein light chain (LC) glycosylation can be detected on routine MASS-FIX testing. Glycosylation is increased in patients with immunoglobulin LC amyloidosis (AL) and rarely changes over the course of a patient’s lifetime. To determine the rates of progression to AL and other plasma cell disorders (PCDs), we used residual serum samples from the Olmsted monoclonal gammopathy of undetermined significance (MGUS) screening cohort. Four-hundred and fourteen patients with known MGUS were tested by MASS-FIX, and 25 (6%) were found to have glycosylated LCs. With a median follow-up of surviving patients of 22.2 years, the 20-year progression rates to a malignant PCD were 67% (95% CI 29%, 84%) and 13% (95% CI 9%, 18%) for patients with and without glycosylated LCs, respectively. The risk of progression was independent of Mayo MGUS risk score. The respective rates of progression to AL at 20 years were 21% (95% CI 0.0%, 38%) and 3% (95% CI 0.6%, 5.5%). In summary, monoclonal LC glycosylation is a potent risk factor for progression to AL, myeloma, and other PCDs, an observation which could lead to earlier diagnoses and potentially reduced morbidity and mortality.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others
References
Pinho SS, Reis CA. Glycosylation in cancer: mechanisms and clinical implications. Nat Rev Cancer. 2015;15:540–55.
Bellotti V, Mangione P, Merlini G. Review: immunoglobulin light chain amyloidosis-the archetype of structural and pathogenic variability. J Struct Biol. 2000;130:280–9.
Milani P, Murray DL, Barnidge DR, Kohlhagen MC, Mills JR, Merlini G, et al. The utility of MASS-FIX to detect and monitor monoclonal proteins in the clinic. Am J Hematol. 2017;92:772–9.
Kumar S, Murray D, Dasari S, Milani P, Barnidge D, Madden B, et al. Assay to rapidly screen for immunoglobulin light chain glycosylation: a potential path to earlier AL diagnosis for a subset of patients. Leukemia. 2019;33:254–7.
Kourelis T, Murray DL, Dasari S, Kumar S, Barnidge D, Madden B, et al. MASS-FIX may allow identification of patients at risk for light chain amyloidosis before the onset of symptoms. Am J Hematol. 2018;93:E368–70.
Mills JR, Kohlhagen MC, Willrich MAV, Kourelis T, Dispenzieri A, Murray DL. A universal solution for eliminating false positives in myeloma due to therapeutic monoclonal antibody interference. Blood. 2018;132:670–2.
Mills JR, Kohlhagen MC, Dasari S, Vanderboom PM, Kyle RA, Katzmann JA, et al. Comprehensive assessment of M-proteins using nanobody enrichment coupled to MALDI-TOF mass spectrometry. Clin Chem. 2016;62:1334–44.
Kyle RA, Therneau TM, Rajkumar SV, Larson DR, Plevak MF, Offord JR, et al. Prevalence of monoclonal gammopathy of undetermined significance. N Engl J Med. 2006;354:1362–9.
Dispenzieri A, Katzmann JA, Kyle RA, Larson DR, Melton LJ 3rd, Colby CL, et al. Prevalence and risk of progression of light-chain monoclonal gammopathy of undetermined significance: a retrospective population-based cohort study. Lancet. 2010;375:1721–8.
Putter H, Fiocco M, Geskus RB. Tutorial in biostatistics: competing risks and multi-state models. Stat Med. 2007;26:2389–430.
Rajkumar SV, Kyle RA, Therneau TM, Melton LJ 3rd, Bradwell AR, Clark RJ, et al. Serum free light chain ratio is an independent risk factor for progression in monoclonal gammopathy of undetermined significance. Blood. 2005;106:812–7.
Sidana S, Murray DL, Dasari S, Go R, Willrich MA, Snyder M, et al. Glycosylation of immunoglobulin light chains is highly prevalent in cold agglutinin disease submitted. Am J Hematol. 2020. https://doi.org/10.1002/ajh.25843.
Dwulet FE, O’Connor TP, Benson MD. Polymorphism in a kappa I primary (AL) amyloid protein (BAN). Mol Immunol. 1986;23:73–8.
Toft KG, Sletten K, Husby G. The amino-acid sequence of the variable region of a carbohydrate-containing amyloid fibril protein EPS (immunoglobulin light chain, type lambda). Biol Chem Hoppe Seyler. 1985;366:617–25.
Stevens FJ. Four structural risk factors identify most fibril-forming kappa light chains. Amyloid. 2000;7:200–11.
Dasari S, Theis JD, Vrana JA, Meureta OM, Quint PS, Muppa P, et al. Proteomic detection of immunoglobulin light chain variable region peptides from amyloidosis patient biopsies. J Proteome Res. 2015;14:1957–67.
Connors LH, Jiang Y, Budnik M, Theberge R, Prokaeva T, Bodi KL, et al. Heterogeneity in primary structure, post-translational modifications, and germline gene usage of nine full-length amyloidogenic kappa1 immunoglobulin light chains. Biochemistry. 2007;46:14259–71.
Gudelj I, Lauc G, Pezer M. Immunoglobulin G glycosylation in aging and diseases. Cell Immunol. 2018;333:65–79.
Gertz MA. Immunoglobulin light chain amyloidosis diagnosis and treatment algorithm 2018. Blood Cancer J. 2018;8:44.
Acknowledgements
This research was supported by Mayo Clinic, Rochester, MN, US NIH CA186781, The Predolin Foundation, The JABBS Foundation.
Author information
Authors and Affiliations
Contributions
AD and DM designed the study. AD and DRL analyzed, interpreted the data, and edited the manuscript. BA performed the LC-MS experiments. Other authors contributed intellectual content and review of this manuscript.
Corresponding author
Ethics declarations
Conflict of interest
DM and SD have intellectual property rights to the MASS-FIX assay and patents. The other authors have no conflicts related to this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Dispenzieri, A., Larson, D.R., Rajkumar, S.V. et al. N-glycosylation of monoclonal light chains on routine MASS-FIX testing is a risk factor for MGUS progression. Leukemia 34, 2749–2753 (2020). https://doi.org/10.1038/s41375-020-0940-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41375-020-0940-8
This article is cited by
-
Bringing mass spectrometry into the care of patients with multiple myeloma
International Journal of Hematology (2022)
-
An N-glycosylation hotspot in immunoglobulin κ light chains is associated with AL amyloidosis
Leukemia (2022)
-
Role of mutations and post-translational modifications in systemic AL amyloidosis studied by cryo-EM
Nature Communications (2021)
-
Mass spectrometry for the evaluation of monoclonal proteins in multiple myeloma and related disorders: an International Myeloma Working Group Mass Spectrometry Committee Report
Blood Cancer Journal (2021)
-
MASS-FIX for the detection of monoclonal proteins and light chain N-glycosylation in routine clinical practice: a cross-sectional study of 6315 patients
Blood Cancer Journal (2021)