Abstract
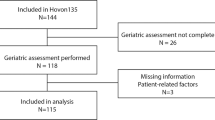
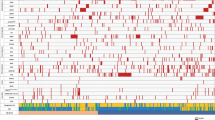
We previously reported the benefit of lomustine addition to conventional chemotherapy in older acute myeloid leukemias with nonadverse chromosomal aberrations in the LAM-SA 2007 randomized clinical trial (NCT00590837). A molecular analysis of 52 genes performed in 330 patients included in this trial, 163 patients being treated with lomustine in combination with idarubicin and cytarabine and 167 without lomustine, identified 1088 mutations with an average of 3.3 mutations per patient. NPM1, FLT3, and DNMT3A were the most frequently mutated genes. A putative therapeutic target was identified in 178 patients (54%). Among five molecular classifications analyzed, the ELN2017 risk classification has the stronger association with the clinical evolution. Patients not treated with lomustine have an expected survival prognosis in agreement with this classification regarding the overall and event-free survivals. In strong contrast, lomustine erased the ELN2017 classification prognosis. The benefit of lomustine in nonadverse chromosomal aberrations was restricted to patients with RUNX1, ASXL1, TP53, and FLT3-ITDhigh/NPM1WT mutations in contrast to the intermediate and favorable ELN2017 patients. This post-hoc analysis identified a subgroup of fit elderly AML patients with intermediate cytogenetics and molecular markers who may benefit from lomustine addition to intensive chemotherapy.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Juliusson G, Abrahamsson J, Lazarevic V, Antunovic P, Derolf A, Garelius H, et al. Prevalence and characteristics of survivors from acute myeloid leukemia in Sweden. Leukemia. 2017;31:728–31.
Oran B, Weisdorf DJ. Survival for older patients with acute myeloid leukemia: a population-based study. Haematologica. 2012;97:1916–24.
DiNardo CD, Wei AH. How I treat acute myeloid leukemia in the era of new drugs. Blood. 2020;135:85–96.
Grimwade D, Walker H, Harrison G, Oliver F, Chatters S, Harrison CJ, et al. The predictive value of hierarchical cytogenetic classification in older adults with acute myeloid leukemia (AML): analysis of 1065 patients entered into the United Kingdom Medical Research Council AML11 trial. Blood. 2001;98:1312–20.
Xie M, Lu C, Wang J, McLellan MD, Johnson KJ, Wendl MC, et al. Age-related mutations associated with clonal hematopoietic expansion and malignancies. Nat Med. 2014;20:1472–8.
Jaiswal S, Fontanillas P, Flannick J, Manning A, Grauman PV, Mar BG, et al. Age-related clonal hematopoiesis associated with adverse outcomes. N Engl J Med. 2014;371:2488–98.
Genovese G, Kahler AK, Handsaker RE, Lindberg J, Rose SA, Bakhoum SF, et al. Clonal hematopoiesis and blood-cancer risk inferred from blood DNA sequence. N Engl J Med. 2014;371:2477–87.
Dohner H, Estey E, Grimwade D, Amadori S, Appelbaum FR, Buchner T, et al. Diagnosis and management of AML in adults: 2017 ELN recommendations from an international expert panel. Blood. 2017;129:424–47.
Pigneux A, Bene MC, Salmi LR, Dumas PY, Delaunay J, Bonmati C, et al. Improved survival by adding lomustine to conventional chemotherapy for elderly patients with aml without unfavorable cytogenetics: results of the LAM-SA 2007 FILO trial. J Clin Oncol. 2018;36:3203–10.
Gerson SL. Regeneration of O6-alkylguanine-DNA alkyltransferase in human lymphocytes after nitrosourea exposure. Cancer Res. 1988;48:5368–73.
Weiss RB, Tormey DC, Holland F, Weinberg VE, Lesnick G, Perloff M, et al. A randomized trial of postoperative five-versus three-drug chemotherapy after mastectomy: a Cancer and Leukemia Group B (CALGB) study. Recent Results Cancer Res. 1982;80:170–6.
Marsh JC. The effects of cancer chemotherapeutic agents on normal hematopoietic precursor cells: a review. Cancer Res. 1976;36:1853–82.
Patel JP, Gonen M, Figueroa ME, Fernandez H, Sun Z, Racevskis J, et al. Prognostic relevance of integrated genetic profiling in acute myeloid leukemia. N Engl J Med. 2012;366:1079–89.
Lindsley RC, Mar BG, Mazzola E, Grauman PV, Shareef S, Allen SL, et al. Acute myeloid leukemia ontogeny is defined by distinct somatic mutations. Blood. 2015;125:1367–76.
Papaemmanuil E, Gerstung M, Bullinger L, Gaidzik VI, Paschka P, Roberts ND, et al. Genomic classification and prognosis in acute myeloid leukemia. N Engl J Med. 2016;374:2209–21.
Bullinger L, Dohner K, Dohner H. Genomics of acute myeloid leukemia diagnosis and pathways. J Clin Oncol. 2017;35:934–46.
Dastugue N, Payen C, Lafage-Pochitaloff M, Bernard P, Leroux D, Huguet-Rigal F, et al. Prognostic significance of karyotype in de novo adult acute myeloid leukemia. The BGMT group. Leukemia. 1995;9:1491–8.
LaRochelle O, Bertoli S, Vergez F, Sarry JE, Mansat-De Mas V, Dobbelstein S, et al. Do AML patients with DNMT3A exon 23 mutations benefit from idarubicin as compared to daunorubicin? A single center experience. Oncotarget. 2011;2:850–61.
Pabst T, Mueller BU, Zhang P, Radomska HS, Narravula S, Schnittger S, et al. Dominant-negative mutations of CEBPA, encoding CCAAT/enhancer binding protein-alpha (C/EBPalpha), in acute myeloid leukemia. Nat Genet. 2001;27:263–70.
Poplin R, Ruano-Rubio V, DePristo M A, Fennell T J, Carneiro M O, Van der Auwera G A, et al. Scaling accurate genetic variant discovery to tens of thousands of samples. bioRxiv 201178; https://doi.org/10.1101/201178.
McKenna A, Hanna M, Banks E, Sivachenko A, Cibulskis K, Kernytsky A, et al. The genome analysis toolkit: a MapReduce framework for analyzing next-generation DNA sequencing data. Genome Res. 2010;20:1297–303.
Eisfeld AK, Kohlschmidt J, Mrozek K, Blachly JS, Walker CJ, Nicolet D, et al. Mutation patterns identify adult patients with de novo acute myeloid leukemia aged 60 years or older who respond favorably to standard chemotherapy: an analysis of alliance studies. Leukemia. 2018;32:1338–48.
Metzeler KH, Herold T, Rothenberg-Thurley M, Amler S, Sauerland MC, Gorlich D, et al. Spectrum and prognostic relevance of driver gene mutations in acute myeloid leukemia. Blood. 2016;128:686–98.
Sebert M, Passet M, Raimbault A, Rahme R, Raffoux E, Sicre de Fontbrune F, et al. Germline DDX41 mutations define a significant entity within adult MDS/AML patients. Blood. 2019;134:1441–4.
Pigneux A, Harousseau JL, Witz F, Sauvezie M, Bene MC, Luquet I, et al. Addition of lomustine to idarubicin and cytarabine improves the outcome of elderly patients with de novo acute myeloid leukemia: a report from the GOELAMS. J Clin Oncol. 2010;28:3028–34.
Nikolova T, Roos WP, Kramer OH, Strik HM, Kaina B. Chloroethylating nitrosoureas in cancer therapy: DNA damage, repair and cell death signaling. Biochim Biophys Acta Rev Cancer. 2017;1868:29–39.
Whitman SP, Maharry K, Radmacher MD, Becker H, Mrozek K, Margeson D, et al. FLT3 internal tandem duplication associates with adverse outcome and gene- and microRNA-expression signatures in patients 60 years of age or older with primary cytogenetically normal acute myeloid leukemia: a Cancer and Leukemia Group B study. Blood. 2010;116:3622–6.
Batista LF, Roos WP, Christmann M, Menck CF, Kaina B. Differential sensitivity of malignant glioma cells to methylating and chloroethylating anticancer drugs: p53 determines the switch by regulating xpc, ddb2, and DNA double-strand breaks. Cancer Res. 2007;67:11886–95.
Bertoli S, Berard E, Huguet F, Huynh A, Tavitian S, Vergez F, et al. Time from diagnosis to intensive chemotherapy initiation does not adversely impact the outcome of patients with acute myeloid leukemia. Blood. 2013;121:2618–26.
Fournier E, Duployez N, Ducourneau B, Raffoux E, Turlure P, Caillot D, et al. Mutational profile and benefit of gemtuzumab ozogamicin in acute myeloid leukemia. Blood. 2020;135:542–6.
Acknowledgements
We thank physicians from participating centers as well as central and local data managers for collecting and monitoring patients’ data. We thank Filothèque members for the conservation of patients samples; Catherine Lacombe, Rachid Boulghana, Laure Cabaret, Ségolène Diry, Krishshanthi Sinnadurai, and Shari Dini Mohamed.
French Innovative Leukemia Organization
J.-P. Marolleau26, A. Aleme26, F. Orsini-Piocelle27, N. Cadoux27, N. Ifrah28, M. Hunault28, C. Marie28, A. Al Jijakli29, G. Lepeu30, H. Zerazhi30, M. Beyrne30, A. Banos31, S. Labarrere31, E. Deconinck32, M. Peria32, A. El Yamani33, O. Kadiri33, B. Choufi34, M. Brument34, A. Pigneux35, T. Leguay35, P.-Y. Dumas35, C. Berthou36, G. Guillerm36, G. Drugmanne36, O. Tournilhac37, G. Roy37, B. Audhuy38, S. Camara38, D. Caillot39, M. Grandjean39, J.-Y. Cahn40, C.-E. Bulabois40, B. Fief40, N. Vey41, C. Ladraa41, V. Dorvaux42, M. Hagopian42, N. Fegueux43, C. Fenoll43, V. Sabadash43, M. Ojeda44, C. Haby44, F. Witz45, C. Bonmati45, M. Lhuire45, J. Delaunay46, P. Peterlin46, L. Airiau46, L. Mannone47, I. Touitou47, E. Jourdan48, D. Umuhire48, M. Alexis49, O. Michel49, F. Dreyfus50, D. Bouscary50, A. Cheung50, L. Sanhes51, F. Touhami51, E. Ribas51, M. Puyade52, M.-P. Gallego-Hernanz52, N. Hugon52, C. Himberlin53, L. Maggi53, T. Lamy54, A. Testu54, E. Tavernier55, S. Marchand55, B. Lioure56, C. Kravanja56, L. Benboubker57, D. Nollet57, M. Attal58, C. Recher58, A. Sarry58, A. Lhermitte59, G. Yrica59, D. Schwartz59, N. Le Montagner59, C. Fenoll59, V. Sabadash59, D. Nollet59, L. Auvray59, R. Delepine59, A. Fayault59
Author information
Authors and Affiliations
Consortia
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Members of the French Innovative Leukemia Organization (FILO) are listed below Acknowledgements.
Supplementary information
Rights and permissions
About this article
Cite this article
Largeaud, L., Cornillet-Lefebvre, P., Hamel, JF. et al. Lomustine is beneficial to older AML with ELN2017 adverse risk profile and intermediate karyotype: a FILO study. Leukemia 35, 1291–1300 (2021). https://doi.org/10.1038/s41375-020-01031-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41375-020-01031-1