Abstract
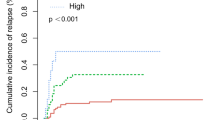
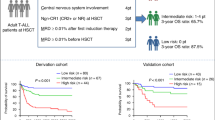
A multicenter retrospective study was performed to explore a prognostic scoring index in order to identify a population who are least likely to benefit from allogeneic hematopoietic cell transplantation (HCT) in patients with relapsed or refractory acute myeloid leukemia (AML). The cohort included 519 patients with AML, who received HCT between 2005 and 2015 at a status of relapse or primary induction failure. Multivariate analysis demonstrated five independent predictors for OS, including C-reactive protein ≥ 1 mg/dL, peripheral blood blast fraction ≥ 20%, poor-risk karyotype, performance status ≥ 2, and bone marrow unrelated donor as a stem cell source. A prognostic scoring index was explored based on these predictors, and successfully separated the cohort into four groups. At 2 years, OS was 47%, 24%, 8%, and 0% for Good (Score 0, 1: n = 118), Intermediate-1 (Score 2: n = 75), Intermediate-2 (Score 3: n = 39), and Poor (Score 4: n = 24), respectively (P < 0.001). The predicting value of the index was confirmed in a validation cohort. Although a further validation study is warranted, the scoring index may be useful to predict survival and to identify the population with the lowest survival prior to HCT in patients with relapsed or refractory AML.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Wong R, Shahjahan M, Wang X, Thall PF, De Lima M, Khouri I, et al. Prognostic factors for outcomes of patients with refractory or relapsed acute myelogenous leukemia or myelodysplastic syndromes undergoing allogeneic progenitor cell transplantation. Biol Blood Marrow Transpl. 2005;11:108–14.
Oyekunle AA, Kröger N, Zabelina T, Ayuk F, Schieder H, Renges H, et al. Allogeneic stem-cell transplantation in patients with refractory acute leukemia: a long-term follow-up. Bone Marrow Transpl. 2006;37:45–50.
Duval M, Klein JP, He W, Cahn JY, Cairo M, Camitta BM, et al. Hematopoietic stem-cell transplantation for acute leukemia in relapse or primary induction failure. J Clin Oncol. 2010;28:3730–8.
Watanabe N, Takahashi Y, Matsumoto K, Hama A, Muramatsu H, Doisaki S, et al. Prognostic factors for outcomes of pediatric patients with refractory or relapsed acute leukemia undergoing allogeneic progenitor cell transplantation. Biol Blood Marrow Transpl. 2011;17:516–23.
Medeot M, Tiribelli M, Patriarca F, Sperotto A, Geromin A, Fanin R. Factors affecting outcome of allogeneic stem cell transplantation as salvage in patients with acute myeloid leukemia primary refractory to intensive induction therapy. Am J Hematol. 2014;89:933–4.
Hemmati PG, Terwey TH, Na IK, Jehn CF, le Coutre P, Vuong LG, et al. Allogeneic stem cell transplantation for refractory acute myeloid leukemia: a single center analysis of long-term outcome. Eur J Haematol. 2015;95:498–506.
Liu N, Ning HM, Hu LD, Jiang M, Xu C, Hu JW, et al. Outcome of myeloablative allogeneic peripheral blood hematopoietic stem cell transplantation for refractory/relapsed AML patients in NR status. Leuk Res. 2015;39:1375–81.
Ogawa H, Ikegame K, Daimon T, Uchida N, Fukuda T, Kakihana K, et al. Impact of pretransplant leukemic blast% in bone marrow and peripheral blood on transplantation outcomes of patients with acute myeloid leukemia undergoing allogeneic stem cell transplantation in non-CR. Bone Marrow Transpl. 2018;53:478–82.
Biggs JC, Horowitz MM, Gale RP, Ash RC, Atkinson K, Helbig W, et al. Bone marrow transplants may cure patients with acute leukemia never achieving remission with chemotherapy. Blood. 1992;80:1090–3.
Brown RA, Wolff SN, Fay JW, Pineiro L, Collins RH Jr, Lynch JP, et al. High-dose etoposide, cyclophosphamide, and total body irradiation with allogeneic bone marrow transplantation for patients with acute myeloid leukemia in untreated first relapse: a study by the North American Marrow Transplant Group. Blood. 1995;85:1391–5.
Brown RA, Wolff SN, Fay JW, Pineiro L, Collins RH Jr, Lynch JP, et al. High-dose etoposide, cyclophosphamide and total body irradiation with allogeneic bone marrow transplantation for resistant acute myeloid leukemia: a study by the North American Marrow Transplant Group. Leuk Lymphoma. 1996;22:271–7.
Greinix HT, Reiter E, Keil F, Fischer G, Lechner K, Dieckmann K, et al. Leukemia-free survival and mortality in patients with refractory or relapsed acute leukemia given marrow transplants from sibling and unrelated donors. Bone Marrow Transpl. 1998;21:673–8.
Godder KT, Hazlett LJ, Abhyankar SH, Chiang KY, Christiansen NP, Bridges KD, et al. Partially mismatched related-donor bone marrow transplantation for pediatric patients with acute leukemia: younger donors and absence of peripheral blasts improve outcome. J Clin Oncol. 2000;18:1856–66.
Goldman FD, Rumelhart SL, DeAlacron P, Holida MD, Lee NF, Miller J, et al. Poor outcome in children with refractory/relapsed leukemia undergoing bone marrow transplantation with mismatched family member donors. Bone Marrow Transpl. 2000;25:943–8.
Michallet M, Thomas X, Vernant JP, Kuentz M, Socié G, Espérou-Bourdeau H, et al. Long-term outcome after allogeneic hematopoietic stem cell transplantation for advanced stage acute myeloblastic leukemia: a retrospective study of 379 patients reported to the Société Française de Greffe de Moelle (SFGM). Bone Marrow Transpl. 2000;26:1157–63.
Tabata M, Satake A, Okura N, Yamazaki Y, Toda A, Nishioka K, et al. Long-term outcome after allogeneic bone marrow transplantation for hematological malignancies with non-remission status. Results of a single-center study of 24 patients. Ann Hematol. 2002;81:582–7.
Fung HC, Stein A, Slovak Ml, O’donnell MR, Snyder DS, Cohen S, et al. A long-term follow-up report on allogeneic stem cell transplantation for patients with primary refractory acute myelogenous leukemia: impact of cytogenetic characteristics on transplantation outcome. Biol Blood Marrow Transpl. 2003;9:766–71.
Nemecek ER, Gooley TA, Woolfrey AE, Carpenter PA, Matthews DC, Sanders JE. Outcome of allogeneic bone marrow transplantation for children with advanced acute myeloid leukemia. Bone Marrow Transpl. 2004;34:799–806.
Zhang WP, Yang D, Song XM, Ni X, Chen J, Chen L, et al. Allogeneic peripheral blood stem cell transplantation is a promising and safe choice for the treatment of refractory/relapsed acute myelogenous leukemia, even with a higher leukemia burden. Biol Blood Marrow Transpl. 2013;19:653–60.
Kurosawa S, Yakushijin K, Yamaguchi T, Atsuta Y, Nagamura-Inoue T, Akiyama H, et al. Recent decrease in non-relapse mortality due to GVHD and infection after allogeneic hematopoietic cell transplantation in non-remission acute leukemia. Bone Marrow Transpl. 2013;48:1198–204.
Tischer J, Stemmler HJ, Engel N, Hubmann M, Fritsch S, Prevalsek D, et al. Feasibility of clofarabine cytoreduction followed by haploidentical hematopoietic stem cell transplantation in patients with relapsed or refractory advanced acute leukemia. Ann Hematol. 2013;92:1379–88.
Chen GL, Liu H, Zhang Y, Thomas J, Ross M, Wang ES, et al. Early versus late preemptive allogeneic hematopoietic cell transplantation for relapsed or refractory acute myeloid leukemia. Biol Blood Marrow Transpl. 2014;20:1369–74.
Jabbour E, Daver N, Champlin R, Mathisen M, Oran B, Ciurea S, et al. Allogeneic stem cell transplantation as initial salvage for patients with acute myeloid leukemia refractory to high-dose cytarabine-based induction chemotherapy. Am J Hematol. 2014;89:395–8.
Tang W, Fan X, Wang L, Hu J. Busulfan and fludarabine conditioning regimen given at hematological nadir of cytoreduction fludarabine, cytarabine, and idarubicin chemotherapy in patients with refractory acute myeloid leukemia undergoing allogeneic stem cell transplantation: a single arm pilot consort study. Med (Baltim). 2015;94:e706.
Decroocq J, Itzykson R, Vigouroux S, Michallet M, Yakoub-Agha I, Huynh A, et al. Similar outcome of allogeneic stem cell transplantation after myeloablative and sequential conditioning regimen in patients with refractory or relapsed acute myeloid leukemia: A study from the Société Francophone de Greffe de Moelle et de Thérapie Cellulaire. Am J Hematol. 2018;93:416–23.
Mohty M, Malard F, Blaise D, Milpied N, Socié G, Huynh A, et al. Sequential regimen of clofarabine, cytosine arabinoside and reduced-intensity conditioned transplantation for primary refractory acute myeloid leukemia. Haematologica. 2017;102:184–91.
NCCN Clinical Practice Guidelines in Oncology. Acute Myeloid Leukemia. Version 2. 2012.
Sorror ML, Maris MB, Storb R, Baron F, Sandmaier BM, Maloney DG, et al. Hematopoietic cell transplantation (HCT)-specific comorbidity index: a new tool for risk assessment before allogeneic HCT. Blood. 2005;106:2912–9.
Bacigalupo A, Ballen K, Rizzo D, Giralt S, Lazarus H, Ho V, et al. Defining the intensity of conditioning regimens: working definitions. Biol Blood Marrow Transpl. 2009;15:1628–33.
Przepiorka D, Weisdorf D, Martin P, Klingemann HG, Beatty P, Hows J, et al. 1994 Consensus Conference on Acute GVHD Grading. Bone Marrow Transpl. 1995;15:825–8.
Shulman HM, Sullivan KM, Weiden PL, McDonald GB, Striker GE, Sale GE, et al. Chronic graft-versus-host syndrome in man. A long-term clinicopathologic study of 20 Seattle patients. Am J Med. 1980;69:204–17.
Kanda Y. Investigation of the freely available easy-to-use software ‘EZR’ for medical statistics. Bone Marrow Transpl. 2013;48:452–8.
Armand P, Gibson CJ, Cutler C, Ho VT, Koreth J, Alyea EP, et al. A disease risk index for patients undergoing allogeneic stem cell transplantation. Blood. 2012;120:905–13.
Armand P, Kim HT, Cutler CS, Ho VT, Koreth J, Ritz J, et al. A prognostic score for patients with acute leukemia or myelodysplastic syndromes undergoing allogeneic stem cell transplantation. Biol Blood Marrow Transpl. 2008;14:28–35.
Akı ŞZ, Suyanı E, Bildacı Y, Çakar MK, Baysal NA, Sucak GT. Prognostic role of pre-transplantation serum C-reactive protein levels in patients with acute leukemia undergoing myeloablative allogeneic stem cell transplantation. Clin Transpl. 2012;26:E513–521.
Artz AS, Logan B, Zhu X, Akpek G, Bufarull RM, Gupta V, et al. The prognostic value of serum C-reactive protein, ferritin, and albumin prior to allogeneic transplantation for acute myeloid leukemia and myelodysplastic syndromes. Haematologica. 2016;101:1426–33.
Yamamoto W, Fujii E, Matsumoto K, Yamamoto E, Aoki J, Tanaka M, et al. Prognostic value of pretransplant serum C-reactive protein in patients receiving reduced-intensity conditioning allogeneic hematopoietic stem cell transplantation. Int J Hematol. 2016;103:444–52.
Kawase T, Matsuo K, Kashiwase K, Inoko H, Saji H, Ogawa S, et al. HLA mismatch combinations associated with decreased risk of relapse: implications for the molecular mechanism. Blood. 2009;113:2851–8.
Fleischhauer K, Beelen DW. HLA mismatching as a strategy to reduce relapse after alternative donor transplantation. Semin Hematol. 2016;53:57–64.
Yabe T, Azuma F, Kashiwase K, Matsumoto K, Orihara T, Yabe H, et al. HLA-DPB1 mismatch induces a graft-versus-leukemia effect without severe acute GVHD after single-unit umbilical cord blood transplantation. Leukemia. 2018;32:168–75.
Acknowledgements
We would like to thank all members of KSGCT and Toyohiro Kawano, Aya Nozaki, and Eri Katou, members of the data center.
Author information
Authors and Affiliations
Consortia
Contributions
TT was the primary investigator, managed the study, performed biomedical statistics, and wrote the paper. JK, TI, and YK contributed to managing and supporting the study as a working member of the group. YN, MT, ND, S Fujiwara, SK, MO, ST, TS, TM, S Fujisawa, EM, KM, NA, MG, RW, KS, KU, and NT contributed to collecting research forms as a representative of the institution. SO supervised the study.
Corresponding author
Ethics declarations
Conflict of interest
Dr. Usuki reports grants and personal fees from MSD K.K., Sumitomo Dainippon Pharma, Pfizer Japan, and Celgene Corporation, grants from Astellas Pharma, Otsuka, Kyowa Kirin, GlaxoSmithKline K.K., Sanofi K.K., Shire Japan, SymBio Pharmaceuticals Limited, Daiichi Sankyo, Boehringer-Ingelheim Japan, and Janssen Pharmaceutical K.K., and personal fees from Novartis, Ono Pharmaceutical, Takeda Pharmaceuticals, Chugai Pharmaceutical, Nippon Shinyaku, and Mochida Pharmaceutical. The remaining authors declare that they have no conflict of interest.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Tachibana, T., Kanda, J., Ishizaki, T. et al. Prognostic index for patients with relapsed or refractory acute myeloid leukemia who underwent hematopoietic cell transplantation: a KSGCT multicenter analysis. Leukemia 33, 2610–2618 (2019). https://doi.org/10.1038/s41375-019-0494-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41375-019-0494-9
This article is cited by
-
Allogeneic hematopoietic cell transplantation for patients with acute myeloid leukemia not in remission
Leukemia (2024)
-
Pretransplantation predictors of survival in nonremission acute myeloid leukemia treated with haploidentical transplantation using steroid-based GVHD prophylaxis
Annals of Hematology (2024)
-
Improving prediction accuracy in acute myeloid leukaemia: micro-environment, immune and metabolic models
Leukemia (2021)
-
Prognostic impact of TP53 mutation, monosomal karyotype, and prior myeloid disorder in nonremission acute myeloid leukemia at allo-HSCT
Bone Marrow Transplantation (2021)
-
Pretransplant increasing rate of lactate dehydrogenase as a predictor of transplant outcomes for patients with myeloid hematological malignancies
Bone Marrow Transplantation (2021)