Abstract
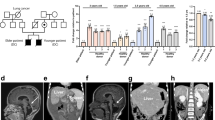
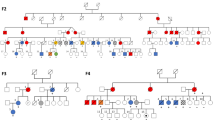
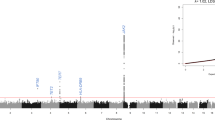
Dyskeratosis congenita (DKC) is a paradigmatic telomere disorder characterized by substantial and premature telomere shortening, bone marrow failure, and a dramatically increased risk of developing myelodysplastic syndrome (MDS) or acute myeloid leukemia (AML). DKC can occur as a late-onset, so-called cryptic form, with first manifestation in adults. Somatic MDS-related mutations are found in up to 35% of patients with acquired aplastic anemia (AA), especially in patients with short telomeres. The aim of our study was to investigate whether cryptic DKC is associated with an increased incidence of MDS-related somatic mutations, thereby linking the accelerated telomere shortening with the increased risk of MDS/AML. Samples from 15 adult patients (median age: 42 years, range: 23–60 years) with molecularly confirmed cryptic DKC were screened using next-generation gene panel sequencing to detect MDS-related somatic variants. Only one of the 15 patients (7%) demonstrated a clinically relevant MDS-related somatic variant. This incidence was dramatically lower than formerly described in acquired AA. Based on our data, we conclude that clonal evolution of subclones carrying MDS-related mutations is not the predominant mechanism for MDS/AML initiation in adult cryptic DKC patients.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Blasco MA. Telomeres and human disease: ageing, cancer and beyond. Nat Rev Genet. 2005;6:611–22.
Brummendorf TH, Balabanov S. Telomere length dynamics in normal hematopoiesis and in disease states characterized by increased stem cell turnover. Leukemia. 2006;20:1706–16.
Greider CW, Blackburn EH. Identification of a specific telomere terminal transferase activity in tetrahymena extracts. Cell. 1985;43:405–13.
Cooper JN, Young NS. Clonality in context: hematopoietic clones in their marrow environment. Blood. 2017;130:2363–72.
Martinez P, Blasco MA. Telomere-driven diseases and telomere-targeting therapies. J Cell Biol. 2017;216:875–87.
Fernandez Garcia MS, Teruya-Feldstein J. The diagnosis and treatment of dyskeratosis congenita: a review. J Blood Med. 2014;5:157–67.
Alter BP, Giri N, Savage SA, Rosenberg PS. Cancer in the National Cancer Institute inherited bone marrow failure syndrome cohort after 15 years of follow-up. Haematologica. 2017;103:30–39.
Alter BP, Giri N, Savage SA, Rosenberg PS. Cancer in dyskeratosis congenita. Blood. 2009;113:6549–57.
Townsley DM, Dumitriu B, Young NS. Bone marrow failure and the telomeropathies. Blood. 2014;124:2775–83.
Schmitt K, Beier F, Panse J, Brummendorf TH. (Pan-)cytopenia as first manifestation of kryptic telomeropathies in adults. Dtsch Med Wochenschr. 2016;141:1578–80.
Bejar R, Stevenson K, Abdel-Wahab O, Galili N, Nilsson B, Garcia-Manero G, et al. Clinical effect of point mutations in myelodysplastic syndromes. N Engl J Med. 2011;364:2496–506.
Sperling AS, Gibson CJ, Ebert BL. The genetics of myelodysplastic syndrome: from clonal haematopoiesis to secondary leukaemia. Nat Rev Cancer. 2017;17:5–19.
Brummendorf TH, Holyoake TL, Rufer N, Barnett MJ, Schulzer M, Eaves CJ, et al. Prognostic implications of differences in telomere length between normal and malignant cells from patients with chronic myeloid leukemia measured by flow cytometry. Blood. 2000;95:1883–90.
Jaiswal S, Fontanillas P, Flannick J, Manning A, Grauman PV, Mar BG, et al. Age-related clonal hematopoiesis associated with adverse outcomes. N Engl J Med. 2014;371:2488–98.
Steensma DP, Bejar R, Jaiswal S, Lindsley RC, Sekeres MA, Hasserjian RP, et al. Clonal hematopoiesis of indeterminate potential and its distinction from myelodysplastic syndromes. Blood. 2015;126:9–16.
Stanley N, Olson TS, Babushok DV. Recent advances in understanding clonal haematopoiesis in aplastic anaemia. Br J Haematol. 2017;177:509–25.
Park HS, Park SN, Im K, Kim SM, Kim JA, Hwang SM, et al. Telomere length and somatic mutations in correlation with response to immunosuppressive treatment in aplastic anaemia. Br J Haematol. 2017;178:603–15.
Kulasekararaj AG, Jiang J, Smith AE, Mohamedali AM, Mian S, Gandhi S, et al. Somatic mutations identify a subgroup of aplastic anemia patients who progress to myelodysplastic syndrome. Blood. 2014;124:2698–704.
Beier F, Balabanov S, Buckley T, Dietz K, Hartmann U, Rojewski M, et al. Accelerated telomere shortening in glycosylphosphatidylinositol (GPI)-negative compared with GPI-positive granulocytes from patients with paroxysmal nocturnal hemoglobinuria (PNH) detected by proaerolysin flow-FISH. Blood. 2005;106:531–3.
Werner B, Beier F, Hummel S, Balabanov S, Lassay L, Orlikowsky T, et al. Reconstructing the in vivo dynamics of hematopoietic stem cells from telomere length distributions. eLife 2015;4:e08687.
Beier F, Masouleh BK, Buesche G, Ventura Ferreira MS, Schneider RK, Ziegler P, et al. Telomere dynamics in patients with del (5q) MDS before and under treatment with lenalidomide. Leuk Res. 2015 S0145-2126(15)30380-5.
Brummendorf TH, Maciejewski JP, Mak J, Young NS, Lansdorp PM. Telomere length in leukocyte subpopulations of patients with aplastic anemia. Blood. 2001;97:895–900.
Weidner CI, Lin Q, Birkhofer C, Gerstenmaier U, Kaifie A, Kirschner M, et al. DNA methylation in PRDM8 is indicative for dyskeratosis congenita. Oncotarget. 2016;7:10765–72.
Graubert TA, Shen D, Ding L, Okeyo-Owuor T, Lunn CL, Shao J, et al. Recurrent mutations in the U2AF1 splicing factor in myelodysplastic syndromes. Nat Genet. 2011;44:53–57.
Walter MJ, Shen D, Ding L, Shao J, Koboldt DC, Chen K, et al. Clonal architecture of secondary acute myeloid leukemia. N Engl J Med. 2012;366:1090–8.
Shirai CL, Ley JN, White BS, Kim S, Tibbitts J, Shao J, et al. Mutant U2AF1 expression alters hematopoiesis and pre-mRNA splicing in vivo. Cancer Cell. 2015;27:631–43.
Jongmans MC, Verwiel ET, Heijdra Y, Vulliamy T, Kamping EJ, Hehir-Kwa JY, et al. Revertant somatic mosaicism by mitotic recombination in dyskeratosis congenita. Am J Hum Genet. 2012;90:426–33.
Perdigones N, Perin JC, Schiano I, Nicholas P, Biegel JA, Mason PJ, et al. Clonal hematopoiesis in patients with dyskeratosis congenita. Am J Hematol. 2016;91:1227–33.
Acknowledgements
We are thankful to Lucia Vankann, Kristina Feldberg and Stephan Klinkenberg for their excellent technical assistance. We thank Kim Kricheldorf for the excellent administration of the “Aachen Telomeropathy Registry”. This work was partly supported by a grant from “Lichterzellen e.V.” (THB).
Author contributions
MK: Performed the experiments, analyzed and interpreted the data and wrote the manuscript. AM: Performed the experiments and analyzed the data. MW: Provided patient samples, clinical data and interpreted the data. MSVF, A-SB, IH: Performed the experiments and interpreted the data. WB, MK, MR, SC, JB, MS, JB, MPR, CMW: Provided patient samples, clinical data and interpreted the data. SK, MB, IK, MS: Performed the experiments, analyzed and interpreted the data. THB co-planned the study, analyzed and interpreted the data and wrote the manuscript; FB conceived and planned the study design, interpreted the data, and wrote the manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
These authors contributed equally: Tim H. Brümmendorf, Fabian Beier.
Electronic supplementary material
Rights and permissions
About this article
Cite this article
Kirschner, M., Maurer, A., Wlodarski, M.W. et al. Recurrent somatic mutations are rare in patients with cryptic dyskeratosis congenita. Leukemia 32, 1762–1767 (2018). https://doi.org/10.1038/s41375-018-0125-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41375-018-0125-x
This article is cited by
-
Pediatric Germline Predisposition to Myeloid Neoplasms
Current Hematologic Malignancy Reports (2022)
-
Towards personalized medicine with iPS cell technology: a case report of advanced systemic mastocytosis with associated eosinophilia
Annals of Hematology (2022)
-
Transient elastography in adult patients with cryptic dyskeratosis congenita reveals subclinical liver fibrosis: a retrospective analysis of the Aachen telomere biology disease registry
Orphanet Journal of Rare Diseases (2021)
-
Early and late stage MPN patients show distinct gene expression profiles in CD34+ cells
Annals of Hematology (2021)
-
PRDM8 reveals aberrant DNA methylation in aging syndromes and is relevant for hematopoietic and neuronal differentiation
Clinical Epigenetics (2020)