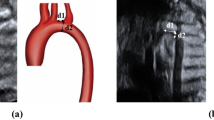
Abstract
Coarctation of the aorta (CoA) is a ductus arteriosus (DA)-dependent form of congenital heart disease (CHD) characterized by narrowing in the region of the aortic isthmus. CoA is a challenging diagnosis to make prenatally and is the critical cardiac lesion most likely to go undetected on the pulse oximetry-based newborn critical CHD screen. When undetected CoA causes obstruction to blood flow, life-threatening cardiovascular collapse may result, with a high burden of morbidity and mortality. Hemodynamic monitoring practices during DA closure (known as an “arch watch”) vary across institutions and existing tools are often insensitive to developing arch obstruction. Novel measures of tissue oxygenation and oxygen deprivation may improve sensitivity and specificity for identifying evolving hemodynamic compromise in the newborn with CoA. We explore the benefits and limitations of existing and new tools to monitor the physiological changes of the aorta as the DA closes in infants at risk of CoA.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Mai CT, Isenburg JL, Canfield MA, Meyer RE, Correa A, Alverson CJ, et al. National population-based estimates for major birth defects, 2010-2014. Birth Defects Res. 2019;111:1420–35.
Hede SV, DeVore G, Satou G, Sklansky M. Neonatal management of prenatally suspected coarctation of the aorta. Prenat Diagn. 2020;40:942–8.
Eckersley L, Sadler L, Parry E, Finucane K, Gentles T. Timing of diagnosis affects mortality in critical congenital heart disease. Arch Dis Child. 2016;101:516–20.
Franklin O, Burch M, Manning N, Sleeman K, Gould S, Archer N. Prenatal diagnosis of coarctation of the aorta improves survival and reduces morbidity. Heart. 2002;87:67–9.
Lannering K, Bartos M, Mellander M. Late diagnosis of coarctation despite prenatal ultrasound and postnatal pulse oximetry. Pediatrics. 2015;136:e406–12.
Chang RKR, Gurvitz M, Rodriguez S. Missed diagnosis of critical congenital heart disease. Arch Pediatr Adolesc Med. 2008;162:969–74.
Maskatia SA, Kwiatkowski D, Bhombal S, Davis AS, McElhinney DB, Tacy TA, et al. A fetal risk stratification pathway for neonatal aortic coarctation reduces medical exposure. J Pediatr. 2021;237:102–8.e3.
Quartermain MD, Pasquali SK, Hill KD, Goldberg DJ, Huhta JC, Jacobs JP, et al. Variation in prenatal diagnosis of congenital heart disease in infants. Pediatrics. 2015;136:e378–85.
Geggel RL. Coarctation of the aorta: delay in diagnosis and referral basis from infancy to adulthood. J Pediatr. 2022;242:57–62.
Rudolph AM. Distribution and regulation of blood flow in the fetal and neonatal lamb. Circ Res. 1985;57:811–21.
Moon-Grady AJ, Donofrio MT, Gelehrter S, Hornberger L, Kreeger J, Lee W, et al. Guidelines and recommendations for performance of the fetal echocardiogram: an update from the american society of echocardiography. J Am Soc Echocardiogr. 2023;36:679–723.
Familiari A, Morlando M, Khalil A, Sonesson SE, Scala C, Rizzo G, et al. Risk factors for coarctation of the aorta on prenatal ultrasound: a systematic review and meta-analysis. Circulation. 2017;135:772–85.
DeVore GR, Haxel C, Satou G, Sklansky M, Pelka MJ, Jone PN, et al. Improved detection of coarctation of the aorta using speckle-tracking analysis of fetal heart on last examination prior to delivery. Ultrasound Obstet Gynecol. 2021;57:282–91.
Ailes EC, Gilboa SM, Honein MA, Oster ME. Estimated number of infants detected and missed by critical congenital heart defect screening. Pediatrics. 2015;135:1000–8.
Peterson C, Ailes E, Riehle-Colarusso T, Oster ME, Olney RS, Cassell CH, et al. Late detection of critical congenital heart disease among US infants: estimation of the potential impact of proposed universal screening using pulse oximetry. JAMA Pediatr. 2014;168:361–70.
Liberman RF, Getz KD, Lin AE, Higgins CA, Sekhavat S, Markenson GR, et al. Delayed diagnosis of critical congenital heart defects: Trends and associated factors. Pediatrics. 2014;134.
Mahle WT, Newburger JW, Matherne GP, Smith FC, Hoke TR, Koppel R, et al. Role of pulse oximetry in examining newborns for congenital heart disease: a scientific statement from the American Heart Association and American Academy of Pediatrics. Circulation. 2009;120:447–58.
Martin GR, Ewer AK, Gaviglio A, Hom LA, Saarinen A, Sontag M, et al. Updated strategies for pulse oximetry screening for critical congenital heart disease. Pediatrics. 2020;146:e20191650.
Hoffman JIE. The challenge in diagnosing coarctation of the aorta. Cardiovascular J Afr. 2018;29:252–5.
Kemper A, Mahle W, Martin G, Cooley WC, Kumar P, Morrow WR, et al. Strategies for Implementing Screening for Critical Congenital Heart Disease. Pediatrics. 2011;128:e1259–67.
Arya B, Maskatia SA. Coarctation of the aorta: Prenatal assessment, postnatal management and neonatal outcomes. Semin Perinatol. 2022;46:151584.
Alpert BS, Quinn D, Gallick D. Oscillometric blood pressure: a review for clinicians. J Am Soc Hypertension. 2014;8:930–8.
Kuck K, Baker PD. Perioperative noninvasive blood pressure monitoring. Anesthesia Analgesia. 2017;127:408–11.
Dasdani S, Aliaga S, Laughon M, Warner D, Price W. Factors influencing the accuracy of noninvasive blood pressure measurements in NICU infant. Am J Perinatol. 2015;32:639–44.
O’Shea J, Dempsey E. A comparison of blood pressure measurements in newborns. Am J Perinatol. 2009;26:113–6.
Shah S, Kaul A, Khandare J, Dhalait S Comparison of Invasive Arterial Blood Pressure Monitoring vs. Non-Invasive Blood Pressure Monitoring in Preterm Infants < 37 Weeks in the Neonatal Intensive Care Unit- A Prospective Observational Study. J Trop Pediatr. 2021;67.
Patankar N, Fernandes N, Kumar K, Manja V, Lakshminrusimha S. Does measurement of four-limb blood pressures at birth improve detection of aortic arch anomalies? J Perinatol. 2016;36:376–80.
Crossland D, Furness J, Abu-Harb M, Sadagopan S, Wren C. Variability of four limb blood pressure in normal neonates. Arch Dis Child Fetal Neonatal Ed. 2004;89:F325–7.
Boelke KL, Hokanson JS. Blood pressure screening for critical congenital heart disease in neonates. Pediatr Cardiol. 2014;35:1349–55.
Ward K, Pryor R, Matson J, Razook J, Thompson W, Elkins R. Delayed detection of coarctation in infancy: implications for timing of newborn follow-up. Pediatrics. 1990;86:972–6.
Wisotzkey BL, Hornik CP, Green AS, Barker PCA. Comparison of invasive and non-invasive pressure gradients in aortic arch obstruction. Cardiol Young-. 2014;25:1348–57.
Giesinger R, McNamara P. Hemodynamic instability in the critically ill neonate: an approach to cardiovascular support based on disease pathophysiology. Semin Perinatol. 2016;40:174–88.
Menahem S, Sehgal A. Doctor please feel my pulses! An aid to diagnosis in the newborn. J Paediatr Child Health. 2016;52:983–90.
Hoffman GM, Ghanayem NS, Kampine JM, Berger S, Mussatto KA, Litwin SB, et al. Venous saturation and the anaerobic threshold in neonates after the Norwood procedure for hypoplastic left heart syndrome. Ann Thorac Surg. 2000;70:1515–20.
Hirsch JC, Charpie JR, Ohye RG, Gurney JG Near-infrared spectroscopy: What we know and what we need to know-A systematic review of the congenital heart disease literature. J Thoracic Cardiovasc Surg. 2009;137.
Harer M, Adegboro C, Richard L, McAdams R. Non-invasive continuous renal tissue oxygenation monitoring to identify preterm neonates at risk for acute kidney injury. Pediatr Nephrol. 2021;36:1617–25.
Marin T, Williams B. Renal oxygenation measured by near-infrared spectroscopy in neonates. Adv Neonatal Care. 2021;21:256–66.
Gagnon MH, Kussman BD, Zhou L, DiNardo JA, Kheir JN. Sensitivity of a next-generation NIRS device to detect low mixed venous oxyhemoglobin saturations in the single ventricle population. Anesth Analg. 2020;131:e138–41.
Rescoe E, Tang X, Perry DA, Sleeper LA, DiNardo JA, Kussman BD, et al. Cerebral near-infrared spectroscopy insensitively detects low cerebral venous oxygen saturations after stage 1 palliation. J Thorac Cardiovasc Surg. 2017;154:1056–62.
Marek J, Fenton M, Khambadkone S Aortic Arch Anomalies: Coarctation of the Aorta and Interrupted Aortic Arch. In: Lai WW, Mertens LL, Cohen MS, Geva T, editors. Echocardiography in Pediatric and Congenital Heart Disease: From Fetus to Adult, 3rd ed. Wiley; (2021).
Park J, Seok HS, Kim SS, Shin H. Photoplethysmogram analysis and applications: an integrative review. Front Physiol. 2022;12:808451.
Granelli ADW, Östman-Smith I. Noninvasive peripheral perfusion index as a possible tool for screening for critical left heart obstruction. Acta Paediatrica, Int J Paediatrics. 2007;96:1455–9.
Siefkes H, Kair L, Tancredi D, Vasquez B, Garcia L, Bedford-Mu C, et al. Oxygen saturation and perfusion index-based enhanced critical congenital heart disease screening. Am J Perinatol. 2020;37:158–65.
Lai Z, Vadlaputi P, Tancredi DJ, Garg M, Koppel RI, Goodman M, et al. Enhanced Critical Congenital Cardiac Disease Screening by Combining Interpretable Machine Learning Algorithms. In: Proceedings of the Annual International Conference of the IEEE Engineering in Medicine and Biology Society, EMBS. Institute of Electrical and Electronics Engineers Inc.; (2021): 1403–6.
Lannering K, Elfvin A, Mellander M. Low false-positive rate of perfusion index as a screening tool for neonatal aortic coarctation. Acta Paediatrica, Int J Paediatrics. 2021;110:1788–94.
Elgendi M, Fletcher R, Liang Y, Howard N, Lovell NH, Abbott D, et al. The use of photoplethysmography for assessing hypertension. NPJ Digital Med. 2019;2:60.
Bartels K, Thiele RH. Advances in photoplethysmography: beyond arterial oxygen saturation. Can J Anesthesia. 2015;62:1313–28.
Allen J, Oates CP, Lees TA, Murray A. Photoplethysmography detection of lower limb peripheral arterial occlusive disease: A comparison of pulse timing, amplitude and shape characteristics. Physiol Meas. 2005;26:811–21.
Kim SH, Song JG, Park JH, Kim JW, Park YS, Hwang GS. Beat-to-beat tracking of systolic blood pressure using noninvasive pulse transit time during anesthesia induction in hypertensive patients. Anesth Analg. 2013;116:94–100.
Palmeri L, Gradwohl G, Nitzan M, Hoffman E, Adar Y, Shapir Y, et al. Photoplethysmographic waveform characteristics of newborns with coarctation of the aorta. J Perinatol. 2017;37:77–80.
Sorensen MW, Sadiq I, Clifford GD, Maher KO, Oster ME. Using pulse oximetry waveforms to detect coarctation of the aorta. Biomed Eng Online. 2020;19:31.
Ward KR, Barbee RW, Reynolds PS, Torres Filho IP, Tiba MH, Torres L, et al. Oxygenation monitoring of tissue vasculature by resonance Raman spectroscopy. Anal Chem. 2007;79:1514–8.
Ward KR, Ivatury RR, Barbee RW, Terner J, Pittman R, Torres Filho IP, et al. Near infrared spectroscopy for evaluation of the trauma patient: a technology review. Resuscitation. 2006;68:27–44.
Tiba MH, Draucker GT, Barbee RW, Terner J, Torres Filho I, Romfh P, et al. Tissue oxygenation monitoring using resonance Raman spectroscopy during hemorrhage. J Trauma Acute Care Surg. 2014;76:402–8.
Perry DA, Salvin JW, Romfh P, Chen P, Krishnamurthy K, Thomson LM, et al. Responsive monitoring of mitochondrial redox states in heart muscle predicts impending cardiac arrest. Sci Transl Med. 2017;9:eaan0117.
Wu M, Pu K, Jiang T, Zhai Q, Ma Z, Ma H, et al. Early label-free analysis of mitochondrial redox states by Raman spectroscopy predicts septic outcomes. J Adv Res. 2021;28:209–19.
Iyengar A, Gaillardetz A, Tighiouart H, Castillo B, Romfh P, Davis JM. Direct measurement of tissue oxygenation in neonates via resonance raman spectroscopy: a pilot study. Neonatology. 2017;112:137–42.
Thomas AR, Kheir JN The Arch Watch Study: An Integrated Evaluation of Hemodynamics in Infants With Suspected Coarctation of the Aorta [Internet]. ClinicalTrials.gov identifier: NCT05880576. (2023). Available from: https://clinicaltrials.gov/study/NCT05880576?cond=coarctation%20of%20the%20aorta&rank=9.
Acknowledgements
We thank Kai-ou Tang, M.A. for her illustration of Figs. 1–3. All figures used with permission from the Cardiac ICU Manual from Boston Children’s Hospital.
Funding
T32 HD 098061, American Academy of Pediatrics Marshall Klaus Perinatal Research Award, Boston Children’s Hospital Translational Research Program Pilot Grant
Author information
Authors and Affiliations
Contributions
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article. AT and JK conceptualized the manuscript. AT, PL, FS, NB, EV, JD, and KF all contributed to the first draft, performed literature review, and revised subsequent drafts. All authors have approved the submitted manuscript and have agreed to be accountable for the contents.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Thomas, A.R., Levy, P.T., Sperotto, F. et al. Arch watch: current approaches and opportunities for improvement. J Perinatol 44, 325–332 (2024). https://doi.org/10.1038/s41372-023-01854-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41372-023-01854-7