Abstract
Objective
We quantified neutralizing SARS-CoV-2 antibody against spike protein (nAb) levels after vaccination and SARS-CoV-2 infection in maternal serum, cord blood, and breast milk and determined whether they correlate with levels of spike protein binding antibody.
Study design
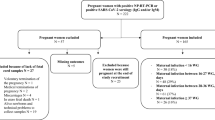
Women (n = 100) were enrolled on admission for delivery. Previous SARS-CoV-2 infection was defined by anti-nucleocapsid antibodies. Levels of nAb and binding antibodies against spike receptor binding domain were measured in maternal blood, cord blood, and milk.
Results
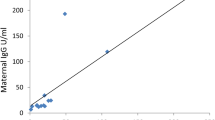
Maternal nAb levels were higher after vaccine and infection than vaccine alone but waned rapidly. Levels of nAb in cord blood and milk correlated with maternal levels and were higher in cord blood than maternal. Spike protein binding antibody levels correlated with nAb.
Conclusion
SARS-CoV-2 vaccination near delivery may boost antibody-mediated immunity in the peripartum period. Neutralizing antibodies are passed transplacentally and into milk. Spike protein binding antibody may be a feasible proxy for nAb.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
Data availability
The datasets generated and analyzed during this study are available from the corresponding author on reasonable request.
References
Panagiotakopoulos L, Myers TR, Gee J, Lipkind HS, Kharbanda EO, Ryan DS, et al. SARS-CoV-2 Infection Among Hospitalized Pregnant Women: Reasons for Admission and Pregnancy Characteristics - Eight U.S. Health Care Centers, March 1-May 30, 2020. MMWR Morb Mortal Wkly Rep. 2020;69:1355–9.
Smith V, Seo D, Warty R, Payne O, Salih M, Chin KL, et al. Maternal and neonatal outcomes associated with COVID-19 infection: a systematic review. PLoS One. 2020;15:e0234187.
Walker KF, O’Donoghue K, Grace N, Dorling J, Comeau JL, Li W, et al. Maternal transmission of SARS-COV-2 to the neonate, and possible routes for such transmission: a systematic review and critical analysis. BJOG. 127: 324-1336.
Moore KM, Suthar MS. Comprehensive analysis of COVID-19 during pregnancy. Biochem Biophys Res Commun. 2021;538:180–6.
Medlow AG, Li JZ, Collier AY, Atyeo C, James KE, Boatin AA, et al. Assessment of maternal and neonatal SARS-CoV-2 viral load, transplacental antibody transfer and placental pathology in pregnancies During the COVID-19 pandemic. JAMA Netw Open. 2020;3:e2030455.
Pace RM, Williams JE, Järvinen KM, Belfort MB, Pace CDW, Lackey KA, et al. Characterization of SARS-CoV-2 RNA, antibodies, and neutralizing capacity in milk produced by women with COVID-19. mBio. 2021;12:e03192–20.
Joseph NT, Dude CM, Verkerke HP, Irby LS, Dunlop AL, Patel RM, et al. Maternal antibody response, neutralizing potency, and placental antibody transfer after severe acute respiratory syndrome Coronavirus 2 (SARS-CoV-2) infection. Obstet Gynecol. 2021;138:189–97.
Atyeo C, Pullen KM, Bordt EA, Fischinger S, Burke J, Michell A, et al. Compromised SARS-CoV-2-specific placental antibody transfer. Cell. 2021;184:628–642.e10.
Flannery DD, Gouma S, Dhudasia MB, Mukhopadhyay S, Pfeifer MR, Woodford EC, et al. Assessment of maternal and neonatal cord blood SARS-CoV-2 antibodies and placent transfer ratios. JAMA Pediatr. 2021;175:594–600.
Wu F, Liu M, Wang A, Lu L, Wang Q, Gu C, et al. Evaluating the association of clinical characteristics with neutralizing antibody levels in patients who have recovered from mild COVID-19 in Shanghai, China. JAMA Intern Med. 2020;180:1356–62.
Katz MH. Neutralizing antibodies against SARS-CoV-2-important questions, unclear answers. JAMA Intern Med. 2020;180:1362.
Gray KJ, Bordt EA, Atyeo C, Deriso E, Akinwunmi B, Young N, et.al. Coronavirus disease 2019 vaccine response in pregnant and lactating women: a cohort study. Am J Obstet Gynecol. 2021; https://doi.org/10.1016/j.ajog.2021.03.023
Collier AY, McMahan K, Yu J, Tostanoski LH, Aguayo R, Ansel J, et al. Immunogenicity of COVID-19 mRNA vaccines in pregnant and lactating women. JAMA. 2021;325:2370–80.
Khoury DS, Cromer D, Reynaldi A, Schlub TE, Wheatley AK, Juno JA, et al. Neutralizing antibody levels are highly predictive of immune protection from symptomatic SARS-CoV-2 infection. Nat Med. 2021;27:1205–11.
Stafford LS, Valcarce V, Henry M, Neu J, Parker L, Mueller M, et al. 3rd. Detection of SARS-CoV-2 IgA and IgG in human milk and breastfeeding infant stool 6 months after maternal COVID-19 vaccination. J Perinatol. 2023;43:775–81.
Cosentino M, Marino F. In response to Detection of SARS-CoV-2 IgA and IgG in human milk and breastfeeding infant stool 6 months after maternal COVID-19 vaccination [letter]. J Perinatol. 2023;43:827.
Keller-Stanislawski B, Englund JA, Kang G, Mangtani P, Neuzil K, Nohynek H, et al. Safety of immunization during pregnancy: a review of the evidence of selected inactivated and live attenuated vaccines. Vaccine. 2014;32:7057e64.
Lönnerdal B. Nestle nutr inst workshop ser. Hum Milk: Bioact Proteins/Pept Funct Prop 2016;86:97–107.
Nolan LS, Parks OB, Good M. A review of the immunomodulating components of maternal breast milk and protection against necrotizing enterocolitis. Nutrients. 2019;12:14.
Huang Y, Borisov O, Kee JJ, Carpp LN, Wrin T, Cai S, et al. Calibration of two validated SARS-CoV-2 pseudovirus neutralization assays for COVID-19 vaccine evaluation. Sci Rep. 2021;11:23921.
Schuh AJ, Satheshkumar PS, Dietz S, Bull-Otterson L, Charles M, Edens C, et al. SARS-CoV-2 convalescent sera binding and neutralizing antibody concentrations compared with COVID-19 vaccine efficacy estimates against symptomatic infection. Microbiol Spectr. 2022;10:e0124722.
Shuaib W, Badaruddin IA, Mansor M, Salleh SA, Hassan MR, Lindong S, et al. SARS-CoV-2 S-RBD IgG and neutralizing antibodies among different categories of health care workers post third dose BNT162b2 mRNA COVID-19 vaccine. Hum Vaccin Immunother. 2023;19:2266931.
Flannery DD, Gouma S, Dhudasia MB, Mukhopadhyay S, Pfeifer MR, Woodford EC, et al. Comparison of maternal and neonatal antibody levels after COVID-19 vaccination vs SARS-CoV-2 infection. JAMA Netw Open. 2022;5:e2240993.
Beharier O, Plitman Mayo R, Raz T, Nahum Sacks K, Schreiber L, Suissa-Cohen Y, et al. Efficient maternal to neonatal transfer of antibodies against SARS-CoV-2 and BNT162b2 mRNA COVID-19 vaccine. J Clin Invest. 2021;131:e150319.
Govindaraj S, Cheedarla N, Cheedarla S, Irby LS, Neish AS, Roback JD, et al. COVID-19 vaccine induced poor neutralization titers for SARS-CoV-2 omicron variants in maternal and cord blood. Front Immunol. 2023;14:1211558.
Young BE, Seppo AE, Diaz N, Rosen-Carole C, Nowak-Wegrzyn A, Cruz Vasquez JM, et al. Association of human milk antibody induction, persistence, and neutralizing capacity with SARS-CoV-2 infection vs mRNA vaccination. JAMA Pediatr. 2022;176:159–68.
Szczygioł P, Łukianowski B, Kościelska-Kasprzak K, Jakuszko K, Bartoszek D, Krajewska M, et al. Antibodies in the breastmilk of COVID-19 recovered women. BMC Pregnancy Childbirth. 2022;22:635.
Golan Y, Ilala M, Li L, Gay C, Hunagund S, Lin CY, et al. Milk antibody response after 3rd COVID-19 vaccine and SARS-CoV-2 infection and implications for infant protection. iScience. 2023;26:107767.
Author information
Authors and Affiliations
Contributions
MK, LR, JC, BW: study design, data analysis, writing manuscript DD: data analysis, writing manuscript KS, MM: data collection, reviewing manuscript CW, CJP, TW, KC: study design, sample analysis, reviewing manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Khalifeh, M., Rubin, L.G., Dayya, D. et al. SARS-CoV-2 neutralizing antibody titers in maternal blood, umbilical cord blood, and breast milk. J Perinatol 44, 28–34 (2024). https://doi.org/10.1038/s41372-023-01843-w
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41372-023-01843-w