Abstract
Objective
Information is needed to guide the design of randomized controlled trials (RCTs) evaluating L-citrulline therapy for premature infants with pulmonary hypertension associated with bronchopulmonary dysplasia (BPD-PH). Based on our single-dose pharmacokinetic study, we evaluated the ability of a multi-dose enteral L-citrulline strategy to achieve a target trough steady-state L-citrulline plasma concentration and its tolerability in premature infants.
Study Design
Plasma L-citrulline concentrations were measured in six premature infants receiving 60 mg/kg L-citrulline every 6 h for 72 h before the first and last L-citrulline doses. L-citrulline concentrations were compared to concentration-time profiles from our previous study.
Results
Target trough plasma L-citrulline concentrations were achieved in 2/6 subjects. No serious adverse events occurred.
Conclusions
Multi-dose L-citrulline was well tolerated. These results will assist in the design of phase II RCTs evaluating L-citrulline dosage strategies to achieve target plasma L-citrulline concentrations in infants at risk for BPD-PH. Clinical trials.gov ID: NCT03542812
Similar content being viewed by others
Introduction
Premature infants with chronic lung disease of prematurity, bronchopulmonary dysplasia (BPD), are at risk of developing pulmonary hypertension (PH) [1,2,3,4,5,6]. It has been estimated that between 8% and 42% of infants with BPD have evidence of PH [1,2,3,4, 6]. Mortality rates for infants with BPD-PH have been reported to be as high as 47% [3, 4, 6,7,8]. In addition, there is a growing appreciation that the individual and societal costs associated with BPD-PH are substantial [9, 10].
Research efforts are needed to develop and evaluate therapies to improve outcomes for infants with BPD-PH. In 2022, the Pediatric Pulmonary Hypertension Network (PPHNet) noted the remarkable contribution of BPD-PH to the spectrum of pediatric PH and highlighted the need for focused research and randomized clinical trials (RCTs) in this understudied group of patients [11]. To date, no RCTs have been performed to evaluate the efficacy of any PH-targeted pharmacotherapy as a treatment to inhibit or reverse the development of BPD-PH [12]. Per FDA guidance, prior to performing a RCT, pharmacokinetic studies are needed to establish doses to be evaluated for efficacy and safety of any drug in a pediatric population [13].
Nitric oxide (NO) is a potent pulmonary vasodilator that can be administered exogenously as inhaled NO (iNO) gas. Oral/enteral supplementation with the NO-L-arginine precursor, L-citrulline, provides a way to increase endogenous NO as a potential treatment for PH [14]. Proof of concept to evaluate enterally administered L-citrulline as a PH-targeted therapy is provided by studies with a newborn piglet model showing that oral L-citrulline increased pulmonary vascular NO production and inhibited both the onset and progressive development of chronic hypoxia-induced PH [15, 16].
Our overarching goal is to evaluate orally/enterally administered L-citrulline as a potential treatment for BPD-PH. As a first step, we recently published data from a phase I study that characterized the PK profile of a single dose of enterally administered L-citrulline in premature infants at risk of developing BPD-PH to derive optimal dosage strategies using simulation-based methodology [17]. The current study aimed to characterize the tolerability and ability to achieve target steady-state trough L-citrulline plasma concentrations when premature infants at risk of developing BPD-PH were treated for 72 h with multiple doses of enteral L-citrulline using a dosage strategy based on simulations from our previous pharmacokinetics study [17]. This information is needed in order to better inform the design of future RCTs that will evaluate safety and efficacy.
Materials/subjects and methods
Patient population
This study was the multiple-dose steady state arm of a phase I study NCT03542812 (PK of L-citrulline in infants at substantial risk of developing PH associated with BPD). The single-dose PK arm of the study has been previously published [17].
We enrolled participants from two Newborn Intensive Care Units (NICUs) in Salt Lake City, UT, the University of Utah Hospital NICU and the Intermountain Medical Center NICU. The institutional review board for human subjects committee at each hospital approved the study protocol. Informed consent was obtained from the legal guardians of all subjects.
Premature neonates born at ≤ 28 weeks gestation were eligible to be enrolled in the study if they required invasive mechanical ventilation or non-invasive positive pressure support (nasal continuous positive airway pressure or high flow nasal cannula ≥ 1 liter per minute) at 32 ± 1 week’s postmenstrual age. Other criteria included (1) the ability to tolerate at least one-half of full-volume oral/gavage tube feedings, using 120 ml/kg/d as full-volume oral/gavage tube feedings, (2) a need for some form of continuous respiratory support for the prior 14 days, and (3) a hemoglobin ≥ 10 mg/dl.
Multiple births and infants with anticipated death prior to hospital discharge were excluded from the study. Also excluded were patients with any known major fetal anomalies, chromosomal aneuploidy, or clinical evidence of congenital heart disease, except for patent ductus arteriosus, atrial septal defect, or ventricular septal defect. Additional exclusion criteria were the presence of any acute illness as defined by a fever >100.4°, vomiting or diarrhea, a urine output < 1 ml/kg/hr., significant feeding intolerance beyond the first week of life, or a history of or known to have liver failure or necrotizing enterocolitis.
Patient monitoring
Vital signs (heart rate, respiratory rate, temperature, blood pressure, and oxygen saturation) and respiratory support (FiO2, mode of support e.g. ventilator, continuous positive airway pressure (CPAP), or high flow nasal cannula (HFNC); and specific settings e.g. cm H2O for CPAP or flow for HFNC) were recorded every 6 h during the 72 h of study drug administration and every 12 h for 24 h post study drug administration.
L-citrulline administration carries a theoretical risk of systemic hypotension. An adverse drop in blood pressure was defined as a decrease in mean arterial blood pressure of more than 25% of baseline blood pressure. Enterally administered medications carry the risk of causing gastro-intestinal disturbances, such as vomiting and diarrhea. Therefore, participants were monitored for clinical evidence of feeding intolerance (including vomiting and diarrhea), necrotizing enterocolitis, and gastrointestinal bleeding. Participants were also monitored for clinical evidence of sepsis. Adverse events were recorded during treatment and within 48 h at the end of treatment. The Principal Investigator determined any potential relationship to L-citrulline treatment. In addition, a data safety monitoring committee reviewed each adverse event and its relationship to L-citrulline. An interim safety analysis was undertaken after the first 3 participants were enrolled and after a total of 6 participants were enrolled.
Drug dosing and sample collection
Each patient received 12 doses of 60 mg/kg enteral L-citrulline. The L-citrulline was donated by Askeplion Pharmaceuticals, Baltimore, MD, and was provided in a powder form that was solubilized in sterile water to achieve 50 mg/ml concentration. Each participant received 1.2 ml/kg (60 mg/kg) of the solubilized L-citrulline every 6 h ± 30 min (4 times a day) for 72 h. The total daily volume of solubilized L-citrulline was 4.8 ml/kg/d for a total daily L-citrulline dose of 240 mg/kg/d. Each dose of L-citrulline was delivered via an indwelling nasogastric feeding tube thirty minutes prior to a feeding.
Two (0.3–0.5 mL) blood samples were collected into EDTA microtainers by heel stick methodology from each infant. The first sample was a pre-dose blood sample collected between 10 min and 6 h before administering the first dose of enteral L-citrulline. The second sample was collected 10 to 30 min before the last (12th) dose of enteral L-citrulline. These sampling times were designed to assess baseline and steady-state trough L-citrulline plasma concentrations.
For each infant, we also collected two urine samples via cotton balls placed in their diapers. The first urine sample was collected the day before administering the first enteral dose of L-citrulline. The second sample was collected between 4 and 8 h following the last (12th) dose of enteral L-citrulline. The urine collection times were designed to assess baseline levels of nitric oxide metabolites (nitrite/nitrate) in the urine and whether levels of nitric oxide metabolites increased in response to 72 h of L-citrulline enteral dosing.
Sample processing and analyses
All blood samples were centrifuged at 1500 g for 5 min at 4 °C. The plasma was separated and stored at −80 °C for later determination of concentrations of the amino acids, L-citrulline (μmol/L) and L-arginine (μmol/L). All amino acid analyses were performed by ARUP Laboratories (Salt Lake City, UT) via Liquid Chromatograph-Tandem Mass Spectrometry (LC-MS/MS) using the Sciex aTRAQTM labeling method and an LC-MS/MS system comprising a Sciex API 4000 triple quadrupole mass spectrometer and a Shimadzu High-Performance Liquid Chromatography System [18]. Amino acids were separated with a C18 chromatographic column and identified using their characteristic retention times and mass transitions. To allow accurate quantification, each analytical run included a calibration curve for each reported amino acid, and internal standards were used to correct for ionization differences and ion suppression.
Each urine sample was aliquoted into two separate tubes (minimum 0.5 ml urine per tube), then stored at −80 °C. One of the aliquoted urine samples was used to determine urine creatinine (Cr). Urine Cr analysis was performed by either ARUP Laboratories (Salt Lake City, UT) or the Central Laboratory at the University of Utah Health Hospital (Salt Lake City, UT).
The second aliquoted urine sample was used to determine urine nitric oxide metabolites, nitrites/nitrates (NOx). For urine NOx analysis, using methods as previously described [19], urine samples were diluted 1:10 with sterile water, then injected (20 μL) into the reaction chamber of a chemiluminescent NO analyzer (Seivers). The reaction chamber contained vanadium (III) chloride in 1 M HCl heated to 90 °C to reduce nitrite and nitrate to NO gas. N2 gas was used to transfer the NO gas to a gas bubble trap where HCl vapor was removed by 1 M NaOH. A standard curve was generated for each analytical run by adding known amounts of NaNO3 to distilled water and assaying as described for the urine samples. Urine NOx was corrected for urine Cr (urine NOx/urine Cr).
Pharmacokinetic analysis
Dosing regimen (using median dose) for the current study was used to obtain simulated concentration-time data and corresponding profiles of L-citrulline using R (version 4.1.0) via package “linpk”. Parameter values for clearance, volume of distribution and absorption rate constant were obtained from a previous study and were fixed at 0.6 L/h, 0.9 L and 0.95, respectively [17]. The concentration time data generated from the simulations were overlaid and visually compared to plasma L-citrulline steady-state trough concentrations obtained from patients in this study.
Statistical analyses
Wilcoxon’s signed rank test was used to compare plasma concentrations of L-citrulline and arginine obtained at baseline to plasma concentrations obtained prior to the 12th L-citrulline dose and to compare values of urine NOx/urine Cr obtained at baseline to those obtained after L-citrulline treatment. A p-value of <0.05 was considered significant.
Results
Subject characteristics
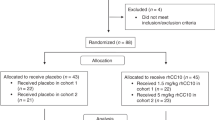
A total of 91 infants born ≤ 28 weeks were screened for eligibility. 62 were ineligible for the following reasons: multiple gestations (n = 27), feeding problems or necrotizing enterocolitis (n = 14), lack of consent form in parent’s language (n = 4), respiratory support below eligibility criteria (n = 4), congenital anomalies (n = 4), sepsis or instability (n = 3), possible liver disease (n = 3), staff unavailable to perform study (n = 2), attending request (n = 1). A total of 6 premature infants were enrolled in this study. The original enrollment target was 18 infants. Slow enrollment and budgetary limitations, which precluded updating the Certificate of Analysis on the L-citrulline product used in this study, necessitated the termination of the study after the sixth study patient was enrolled. The median birth weight was 1050 grams (range 769–1430 g) and the weight at the time of study was 1487.5 grams (range 1330–1880 g) (Table 1). All six patients were receiving gastric human milk feedings, median volume of 142 ml/kg/d (range 132–150 ml/kg/d), and none were receiving parenteral nutrition. None of the subjects received post-pyloric feedings during the study.
Plasma amino acid and urine NOx data
Basal plasma concentrations were obtained between 30 min to 5 ½ hours prior to administering the first dose of L-citrulline, and a second plasma concentration was obtained between 18–27 min prior to the last, 12th, dose of L-citrulline. There was no difference between the baseline L-citrulline plasma concentrations (median 37.5 μmol/L, IQR 20–45 μmol/L), and the trough plasma concentrations obtained prior to the last dose of L-citrulline (median 38 μmol/L, IQR 30.5–53 μmol/L, p = 0.17) for the combined group of six patients. There was also no difference between baseline plasma arginine concentrations (median 75.5 μmol/L, IQR 54.5–114.3 μmol/L), and trough plasma arginine concentrations obtained prior to the last dose of L-citrulline (median 112 μmol/L, IQR 86–161.3 μmol/L p = 0.12) in this study cohort. When considering each individual patient as shown in Fig. 1 A, for five of the six subjects the trough plasma L-citrulline concentration obtained before the last dose of L-citrulline treatment was higher than that of the subject’s baseline plasma L-citrulline concentration. The increase from baseline was variable, ranging from an increase of 10 to 81%. As shown in Fig. 1 B, for four of the subjects, the trough plasma arginine concentration obtained prior to the last dose of L-citrulline treatment was higher than the plasma arginine concentration obtained at baseline for that individual subject. The percent increase from baseline plasma arginine concentration was variable, ranging from an increase of 21 to 215%. Figure 2 illustrates the comparison between the plasma L-citrulline concentration-time profile simulated in the single-dose study and those observed from the subjects in this multi-dose study. Trough L-citrulline plasma concentrations measured at the second time point exceeded 50 μmol/L for 2 subjects. L-citrulline plasma concentrations were excluded for one subject because a protocol deviation occurred that resulted in the second L-citrulline plasma concentration being collected at a timepoint offset by 3 h from the other five subjects.
A Plasma L-citrulline concentrations (μmol/L) of individual subjects collected at baseline and prior to the 12th dose of L-citrulline. B Plasma arginine concentrations (μmol/L) of individual subjects collected at baseline and prior to the 12th dose of L-citrulline. C Urine NOx/Urine Cr levels of individual subjects collected before (baseline) and after the 12th dose of L-citrulline.
Solid black lines represent predicted plasma L-citrulline concentrations. Dotted black lines represent 50 μmol/L and 80 μmol/L concentrations and reflect the target trough plasma concentrations. Black dots are the observed L-citrulline concentrations (μmol/L) collected at study time points (i.e. at baseline and trough prior to the 12th dose of L-citrulline). Note that 2 subjects had the same trough L-citrulline concentration of 53 μmol/L at the second study time point so that dot reflects the data for 2 subjects.
Five of the six premature infants enrolled in this study provided two urine NOx/Cr levels. The first urine sample from these five subjects was collected before administering the first dose of L-citrulline or shortly after the first dose of L-citrulline was given. The first urine sample collected from one patient was lost. All six premature infants enrolled in this study provided urine NOx/Cr levels from urine collected between 6 ¼ and 7 ½ hours after administering the last study dose of L-citrulline. There was no difference between the urine NOx/Cr levels (median 25.8, IQR 22–44) obtained as a baseline and the urine NOx/Cr levels obtained after the last dose of L-citrulline (median 36, IQR 27–46.6) for the combined group of 5 subjects that provided two samples (p = 0.89). When considering values for each of the individual subjects, as shown in Fig. 1 C, only 2 subjects, had a NOx/Cr value obtained after the last dose of L-citrulline treatment that was higher than that subject’s baseline urine NOx/Cr value.
Physiologic parameters, tolerability and safety data
Table 2 summarizes respiratory support at the start and end of the 72 h of L-citrulline treatment for all six subjects. Respiratory support changed slightly, if at all, between the start and end of the study for each of the subjects (Table 2).
All six subjects tolerated gastric feedings throughout the study. None of the subjects developed vomiting, diarrhea, or evidence of necrotizing enterocolitis or gastrointestinal bleeding. No subject was evaluated for sepsis during the study drug treatment.
Figure 3 illustrates the mean BP measurements obtained as a baseline during the 12 h before administering the first L-citrulline and throughout the 72 h of drug administration. Each BP measurement was coordinated with each q 6-hour dose of L-citrulline. Specifically, the BP measurements were obtained during the time of 30 min before to 50 min after each of the 12 doses of L-citrulline. Only one subject experienced a decrease in mean BP to a level more than 25 % below that subject’s baseline. The low BP recording was obtained 30 min after the 3rd dose of L-citrulline. The assessment by the caregivers at the time of the lower BP recording was that the subject was asymptomatic and that no treatment was required. That subject’s BP increased, returned to and remained at acceptable levels for the remainder of the study. The adverse event was reported to the DSMB as a non-serious, mild adverse event, possibly related to the study drug administration. No other adverse events occurred during the study.
Discussion
Premature infants with BPD-PH are an understudied group of patients desperately needing new therapies. The current use of PH-targeted medications in neonates with BPD-PH is off-label. RCTs are needed to evaluate the safety and efficacy of PH-targeted pharmacotherapies in premature infants with or at risk of developing BPD-PH. Prior to performing RCTs, PK studies need to be conducted to guide the choice of doses to be evaluated for safety and efficacy [13]. As a first step in developing oral L-citrulline as a potential therapy for BPD-PH, we recently completed a study that simulated the PK profile of a multi-dosing regimen from data from a single dose of enterally administered L-citrulline in premature infants at risk of developing BPD-PH [17]. The current study showed that when six subjects were given even doses of L-citrulline four times a day, only two subjects (33%) achieved the target trough plasma L-citrulline concentrations of 50–80 μmol/L (8.76–14 μg/ml). Moreover, only one subject (17%) achieved a trough plasma L-citrulline concentration that increased 50–100% above baseline.
Several previously published findings influenced our decision to target steady-state trough L-citrulline plasma concentrations of 50–80 μmol/L in this study. We were aware that investigators interested in developing L-citrulline as a treatment to prevent post-operative PH in infants and children with congenital heart disease undergoing cardiopulmonary bypass had identified plasma L-citrulline concentrations above 37 μmol/L as being protective [20, 21]. A recent study also found that premature infants with BPD-PH had median plasma L-citrulline concentrations of 21 μmol/L, whereas median plasma L-citrulline concentrations were higher, 36 μmol/L, in infants with BPD who did not develop PH [22]. Findings from these studies suggest that achieving plasma L-citrulline concentrations above 37 μmol/L might be needed to prevent various forms of PH in children. Moreover, oral treatment with L-citrulline inhibited PH development in chronically hypoxic piglets who achieved 50–100% increases from their basal plasma L-citrulline concentrations [15, 16]. This latter finding led to our choice to target steady-state trough L-citrulline plasma concentrations at least 50–100% above basal levels of our patient population, using the median basal plasma L-citrulline concentrations of patients in our previous study, 31 μmol/L. Notably, the basal L-citrulline plasma concentrations of the 6 patients in this study, median 37.5 μmol/L, were similar to those in our previous study and comparable to previously published basal levels in pediatric patients [23].
The dosing strategy for this study was based on an optimal design dosage simulation-based methodology from our single-dose pharmacokinetic study [17, 24]. Simulations in our previous study predicted that enterally administering a L-citrulline daily dose of 150 mg/kg/d given once daily to eight times a day would achieve steady-state target trough plasma L-citrulline concentrations of 50–80 μmol/L (8.76–14 μg/ml) in premature infants at 32 ± 1 week postmenstrual age. In order to achieve trough plasma L-citrulline concentrations in the range of 80 μmol/L (14 μg/ml), doses of approximately 240 mg/kg/d are predicted to be needed. We considered practical issues when deciding the dosing frequency. The volume of each dose needed to be small enough to minimize the potential for emesis. In addition, since the long-range intent is that the L-citrulline will be administered for weeks to months, the frequency of dosing needs to not be overly burdensome for the caregiver. Therefore, a total daily dose of 240 mg/kg/d (total daily volume of 4.8 ml/kg/d) was divided into 60 mg/kg doses q 6 h (1.2 ml/kg/dose given 4 x a day) which considered both the desired target trough L-citrulline plasma concentrations and practical considerations of drug administration.
Of note, L-citrulline is endogenously produced from amino acids, including glutamine and proline, by enterocytes in the proximal intestines [25]. It is possible that differences in total daily amounts of dietary protein administered and absorbed by the gastro-intestinal tract might have contributed to the range in patient plasma L-citrulline concentrations measured both at baseline and after 72 h of L-citrulline administration. L-citrulline is considered a non-essential amino acid, so L-citrulline is not present in either infant formulas or parenteral nutrition. In addition, the normal human adult diet contains almost no L-citrulline, so the L-citrulline content in human milk is negligible [26]. Thus, other than the L-citrulline administered every 6 h as part of this study, no patient should have received an exogenous source of L-citrulline that would have influenced their L-citrulline plasma concentrations.
Orally administered L-citrullline has been shown to be highly bioavailable in adults [27]. L-citrulline is absorbed by the intestines via a number of amino acid transporters and then passes through the liver without major metabolism to reach the systemic circulation [25, 26]. The main organ of circulating L-citrulline consumption is the kidney [25, 26]. Approximately 75% of L-citrulline is converted by kidney proximal tubules into arginine, which is subsequently released into the renal vein and the systemic circulation [25, 26]. Some circulating L-citrulline is transported into vascular endothelial cells, including pulmonary arterial endothelial cells, by neutral amino acid transporters and then converted by a two-step enzymatic process into arginine [14, 26]. Less than 1% of an enteral dose of L-citrulline is excreted in the urine [27]. The potential impact from developmental changes on any of the preceding aspects of enterally administered L-citrulline processing is not yet known. However, it should be considered that variability in the bioavailability of the enterally administered L-citrulline, i.e., variability between patients in the amount of L-citrulline absorbed by the gastro-intestinal tract, may have contributed to the variability in degree of increase from baseline L-citrulline plasma concentrations and help explain why the trough increased 50–100% above baseline in only one of the six patients (17%).
Since a sizable amount of circulating L-citrulline is converted in the kidney to L-arginine, it is unsurprising that for most of the patients in the study the change in plasma concentrations of L-arginine paralleled those for L-citrulline. However, unlike L-citrulline, L-arginine is a semi-essential amino acid that must be provided as part of the human diet [28, 29]. This is particularly true for premature infants with a limited ability to produce L-arginine endogenously and reliance on exogenous, i.e. dietary sources, to maintain plasma L-arginine levels that are considered adequate for their needs [29]. Since the volume of human milk feedings ranged from 132–150 ml/kg/d, amounts of exogenously supplied dietary L-arginine might have contributed to the variability in plasma L-arginine concentrations between subjects in this study.
Oral administration of L-citrulline has been shown to increase the rate of plasma NO production in both children and adults [30, 31]. Urinary nitrites and nitrates have been used to reflect systemic NO production [32, 33]. Therefore, it may seem surprising that only two patients in this study had a change in urine NOx/Cr that paralleled increases in plasma concentrations of L-arginine and L-citrulline. Notably, other investigators have been unable to detect significant changes in urine nitrites and nitrates without administering very high doses of oral L-citrulline to adults (3 g L-citrulline twice a day for a week) [34]. Moreover, although rates of NO production increased with L-citrulline administration, plasma NOx concentrations did not change [30]. Both plasma and urine nitrite and nitrate concentrations are known to be confounded by dietary sources, limiting the ability of these measurements to reflect systemic or pulmonary NO production. Consequently, urine NOx/Cr measurements should not be relied on to accurately reflect changes in systemic or pulmonary NO production. Thus, even though we did not find an increase in Urine NOx/Cr with L-citrulline treatment in our group of 6 patients, it should not be concluded that systemic or pulmonary NO production did not increase with L-citrulline treatment in any or all the subjects.
Importantly, this study showed that this fragile patient population well tolerated the L-citrulline dosage strategy used in this study. For example, none of the patients developed evidence of gastrointestinal intolerance with the L-citrulline dosage strategy. Nor did any of the patients experience a decrease in systemic BP that warranted any therapeutic intervention. In addition, with exception of one subject who had a slight increase in respiratory support, the respiratory support was unchanged or reduced during study drug administration. This latter finding is important because neonates with respiratory diseases are at risk of developing pulmonary edema when total daily fluid volumes are increased. Maintaining respiratory stability in our patients in the face of the additional fluid volume required to administer the L-citrulline, 4.8 ml/kg/d in this case, is an important consideration.
We intended to study a population of premature infants at risk of developing BPD-PH. Premature infants born at ≤ 28 weeks gestation are known to be at greater risk of developing BPD than those born at more mature gestational ages [35, 36]. Current definitions of BPD are based on the respiratory support needs at 36 weeks postmenstrual age, with the definition of no BPD being the lack of respiratory support at 36 weeks of postmenstrual age [35, 36]. Therefore, infants were eligible if they had been born at < 28 weeks gestation and required respiratory support on the day of and for 14 days prior to study initiation. We chose to study infants at 32 ± 1 weeks postmenstrual age and not wait until 36 weeks, the postmenstrual age at which BPD is diagnosed. This choice is because our ultimate goal is to perform a RCT to determine whether starting oral L-citrulline treatment at or before 32 weeks postmenstrual age will reduce the percentage of neonates with BPD who have evidence of PH when they are ≥ 36 weeks postmenstrual age.
This study has limitations. One limitation is the small number of patients that we could enroll. Future Phase II studies need to be conducted in a larger number of patients to more completely evaluate the dosing strategies needed to achieve targeted steady-state plasma concentrations. Consistent with the experience of other investigators [37], we believe that one obstacle to successfully enrolling fragile patients into phase I studies like ours is that their parents were reluctant to grant consent for a study that had no direct benefit. Another issue is that none of our patients required invasive respiratory support at the time of study. Thus, our findings may not reflect patients who develop severe BPD. Another limitation is that because we performed the study with premature infants, we used a limited sampling strategy and collected only two blood samples per patient. The total volume of blood sampled during a study in newborns is limited in accordance with IRB guidelines. Meeting these guidelines often necessitates the use of sparse sampling strategies, which poses a major limitation to investigators evaluating pharmacokinetics of drugs in newborns. Limitations in the assays used to determine plasma L-citrulline and L-arginine concentrations may also have impacted our findings. We were also limited by not knowing the actual steady-state L-citrulline concentrations that will achieve therapeutic efficacy. Nor did we include efficacy end points. Indeed, it should be emphasized that no phase III RCT has been performed to evaluate and prove that targeting the NO pathway with any medication, including L-citrulline, is an efficacious therapy for infants with BPD-PH. This phase I study aimed to provide information to better inform the design of future phase II and phase III RCTs.
In summary, this study builds on the data from our previous single-dose PK study conducted in premature infants at risk of developing BPD-PH [17]. Although only two of the six subjects achieved our target trough L-citrulline plasma concentrations, these data can inform the choice of plasma sampling times and doses needed to achieve target L-citrulline plasma concentrations in a future phase II RCT performed in a larger number of subjects. Ultimately, phase III RCTs must be conducted to evaluate whether using an oral L-citrulline dosing strategy that achieves steady-state trough plasma L-citrulline concentrations of 50–80 μmol/L effectively prevents or ameliorates BPD-PH. Prior to performing a phase III RCT, a phase II RCT should be performed to evaluate the safety and provide some pharmacodynamic and preliminary efficacy information. Taken together, the results of this and our previous study provide the critical information needed to design a phase II RCT and take the next step towards evaluating the use of oral L-citrulline as a potential treatment to inhibit BPD-PH in premature infants.
Data availability
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
References
An HS, Bae EJ, Kim GB, Kwon BS, Beak JS, Kim EK, et al. Pulmonary hypertension in preterm infants with bronchopulmonary dysplasia. Korean Circ J. 2010;40:131–6.
Bhat R, Salas AA, Foster C, Carlo WA, Ambalavanan N. Prospective analysis of pulmonary hypertension in extremely low birth weight infants. Pediatrics. 2012;129:e682–689.
Du Y, Yuan L, Zhou JG, Huang XY, Lin SB, Yuan M, et al. Echocardiography evaluation of bronchopulmonary dysplasia-associated pulmonary hypertension: a retrospective observational cohort study. Transl Pediatr. 2021;10:73–82.
Hansmann G, Sallmon H, Roehr CC, Kourembanas S, Austin ED, Koestenberger M, et al. Pulmonary hypertension in bronchopulmonary dysplasia. Pediatr Res. 2021;89:446–55.
Levy PT, Levin J, Leeman KT, Mullen MP, Hansmann G, Kourembanas S. Diagnosis and management of pulmonary hypertension in infants with bronchopulmonary dysplasia. Semin Fetal Neonatal Med. 2022;27:101351.
Weismann CG, Asnes JD, Bazzy-Asaad A, Tolomeo C, Ehrenkranz RA, Bizzarro MJ. Pulmonary hypertension in preterm infants: results of a prospective screening program. J Perinatol. 2017;37:572–7.
Abman SH, Accurso FJ, Bowman CM. Unsuspected cardiopulmonary abnormalities complicating bronchopulmonary dysplasia. Arch Dis Child. 1984;59:966–70.
Fouron JC, Le Guennec JC, Villemant D, Perreault G, Davignon A. Value of echocardiography in assessing the outcome of bronchopulmonary dysplasia of the newborn. Pediatrics. 1980;65:529–35.
Check J, Gotteiner N, Liu X, Su E, Porta N, Steinhorn R, et al. Fetal growth restriction and pulmonary hypertension in premature infants with bronchopulmonary dysplasia. J Perinatol. 2013;33:553–7.
Stuart BD, Sekar P, Coulson JD, Choi SE, McGrath-Morrow SA, Collaco JM. Health-care utilization and respiratory morbidities in preterm infants with pulmonary hypertension. J Perinatol. 2013;33:543–7.
Abman SH, Mullen MP, Sleeper LA, Austin ED, Rosenzweig EB, Kinsella JP, et al. Characterisation of paediatric pulmonary hypertensive vascular disease from the PPHNet Registry. Eur Respir J. 2022;59:203337.
Fike CD, Aschner JL. Pharmacotherapy for Pulmonary Hypertension in Infants with Bronchopulmonary Dysplasia: Past, Present, and Future. Pharmaceuticals (Basel). 2023;16:503–25.
USFDA (United States Department of Health and Human Services Food and Drug Administration). General clinical pharmacology considerations for pediatric studies for drugs including biological products, guidance for industry. 2022, 129532:1–23. https://www.fda.gov/media/129532/download.
Fike CD, Summar M, Aschner JL. L-citrulline provides a novel strategy for treating chronic pulmonary hypertension in newborn infants. Acta Paediatr. 2014;103:1019–26.
Ananthakrishnan M, Barr FE, Summar ML, Smith HA, Kaplowitz M, Cunningham G, et al. L-Citrulline ameliorates chronic hypoxia-induced pulmonary hypertension in newborn piglets. Am J Physiol Lung Cell Mol Physiol. 2009;297:L506–511.
Fike CD, Dikalova A, Kaplowitz MR, Cunningham G, Summar M, Aschner JL. Rescue Treatment with L-citrulline inhibits hypoxia-induced pulmonary hypertension in newborn pigs. Am J Respir Cell Mol Biol. 2015;53:255–64.
Fike CD, Avachat C, Birnbaum AK, Aschner JL, Sherwin CM. Pharmacokinetics of L-citrulline in neonates at risk of developing bronchopulmonary dysplasia-associated pulmonary hypertension. Paediatr Drugs. 2023;25:87–96.
De Biase I, Liu A, Yuzyuk T, Longo N, Pasquali M. Quantitative amino acid analysis by liquid chromatography-tandem mass spectrometry: implications for the diagnosis of argininosuccinic aciduria. Clin Chim Acta. 2015;442:73–74.
O’Connor MG, Suthar D, Vera K, Slaughter JC, Maitre NL, Steele S, et al. Pulmonary hypertension in the premature infant population: Analysis of echocardiographic findings and biomarkers. Pediatr Pulmonol. 2018;53:302–9.
Barr FE, Beverley H, VanHook K, Cermak E, Christian K, Drinkwater D, et al. Effect of cardiopulmonary bypass on urea cycle intermediates and nitric oxide levels after congenital heart surgery. J Pediatr. 2003;142:26–30.
Smith HA, Canter JA, Christian KG, Drinkwater DC, Scholl FG, Christman BW, et al. Nitric oxide precursors and congenital heart surgery: a randomized controlled trial of oral citrulline. J Thorac Cardiovasc Surg. 2006;132:58–65.
Montgomery AM, Bazzy-Asaad A, Asnes JD, Bizzarro MJ, Ehrenkranz RA, Weismann CG. Biochemical Screening for Pulmonary Hypertension in Preterm Infants with Bronchopulmonary Dysplasia. Neonatology. 2016;109:190–4.
Goossens L, Bouvry M, Vanhaesebrouck P, Wuyts B, Van Maele G, Robberecht E. Citrulline levels in a paediatric age group: does measurement on dried blood spots have additional value? Clin Chim Acta. 2011;412:661–4.
Roberts JK, Stockmann C, Balch A, Yu T, Ward RM, Spigarelli MG, et al. Optimal design in pediatric pharmacokinetic and pharmacodynamic clinical studies. Paediatr Anaesth. 2015;25:222–30.
Curis E, Nicolis I, Moinard C, Osowska S, Zerrouk N, Benazeth S, et al. Almost all about citrulline in mammals. Amino Acids. 2005;29:177–205.
Allerton TD, Proctor DN, Stephens JM, Dugas TR, Spielmann G, Irving BA. L-citrulline supplementation: impact on cardiometabolic health. Nutrients 2018;10. https://doi.org/10.3390/nu10070921.
Rouge C, Robert CD, Robbins A, Bacquer OL, Volteau C, Cochetiere MDL, et al. Manipulation of citrulline availability in humans. Am J Physiol Gastrointest Liver Physiol. 2007;293:G1061–G1067.
Cynober LA. Plasma amino acid levels with a note on membrane transport: characteristics, regulation, and metabolic significance. Nutrition. 2002;18:761–6.
Wu G, Jaeger LA, Bazer FW, Rhoads JM. Arginine deficiency in preterm infants: biochemical mechanisms and nutritional implications. J Nutr Biochem. 2004;15:442–51.
El-Hattab AW, Hsu JW, Emrick LT, Wong LJ, Craigen WJ, Jahoor F, et al. Restoration of impaired nitric oxide production in MELAS syndrome with citrulline and arginine supplementation. Mol Genet Metab. 2012;105:607–14.
El-Hattab AW, Emrick LT, Hsu JW, Chanprasert S, Almannai M, Craigen WJ, et al. Impaired nitric oxide production in children with MELAS syndrome and the effect of arginine and citrulline supplementation. Mol Genet Metab. 2016;117:407–12.
Ballard PL, Keller RL, Black DM, Durand DJ, Merrill JD, Eichenwald EC, et al. Inhaled nitric oxide increases urinary nitric oxide metabolites and cyclic guanosine monophosphate in premature infants: relationship to pulmonary outcome. Am J Perinatol. 2015;32:225–32.
Kurioka S, Koshimura K, Sugitani M, Murakami Y, Nishiki M, Kato Y. Analysis of urinary nitric oxide metabolites in healthy subjects. Endocr J. 1999;46:421–8.
Schwedhelm E, Maas R, Freese R, Jung D, Lukacs Z, Jambrecina A, et al. Pharmacokinetic and pharmacodynamic properties of oral L-citrulline and L-arginine: impact on nitric oxide metabolism. Br J Clin Pharm. 2008;65:51–59.
Higgins RD, Jobe AH, Koso-Thomas M, Bancalari E, Viscardi RM, Hartert TV, et al. Bronchopulmonary Dysplasia: Executive Summary of a Workshop. J Pediatr. 2018;197:300–8.
Jensen EA, Dysart K, Gantz MG, McDonald S, Bamat NA, Keszler M, et al. The diagnosis of bronchopulmonary dysplasia in very preterm infants. An Evidence-based Approach. Am J Respir Crit Care Med. 2019;200:751–9.
Jackson W, Gonzalez D, Smith PB, Ambalavanan N, Atz AM, Sokol GM, et al. Safety of sildenafil in extremely premature infants: a phase I trial. J Perinatol. 2022;42:31–36.
Acknowledgements
We thank Asklepion Pharmaceuticals for the generous gift of the oral L-citrulline used in this study. We also thank the Newborn Clinical Research Nurse Coordinators at the University of Utah and Intermountain Medical Center for their help in enrolling patients and performing the study.
Funding
This work was supported by National Heart, Lung, and Blood Institute Grant R34-HL-142995 (CDF).
Author information
Authors and Affiliations
Contributions
CDF, JLA, and CMS contributed to the conception and design of the study. Data collection was performed by CDF. Formal data analysis was performed by CDF, AKB, CA, and CMS. CDF wrote the first draft of the abstract. All authors reviewed and edited drafts of the manuscript and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
CDF and JLA are inventors on a patent at Vanderbilt University Medical Center that has been licensed to Asklepion Pharmaceuticals for the “Therapeutic treatment of bronchopulmonary dysplasia.”
Ethics approval
All procedures performed in this study involving human participants were in accordance with the 1964 Helsinki Declaration and its later amendments. The study design was approved by the institutional review boards of The University of Utah and Intermountain Healthcare. All participants or legal guardians of participants in the clinical trial included in these analyses provided written informed consent.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Fike, C.D., Aschner, J.L., Avachat, C. et al. Multi-dose enteral L-citrulline administration in premature infants at risk of developing pulmonary hypertension associated with bronchopulmonary dysplasia. J Perinatol 44, 280–287 (2024). https://doi.org/10.1038/s41372-023-01809-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41372-023-01809-y