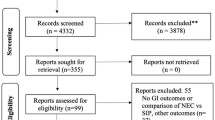
Abstract
Objective
To describe the epidemiology, risk factors, and timing of spontaneous intestinal perforation (SIP) among infants born at 22–24 weeks’ gestational age (GA).
Study design
Observational cohort study among infants born at 22–24 weeks’ GA in 446 neonatal intensive care units.
Results
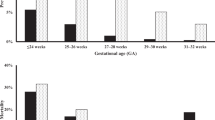
We identified 9712 infants, of whom 379 (3.9%) developed SIP. SIP incidence increased with decreasing GA (P < 0.001). Antenatal magnesium (odds ratio (OR) 1.42; 95% confidence interval (CI), 1.09–1.85), antenatal indomethacin (OR 1.40; 95% CI, 1.06–1.85), postnatal indomethacin (OR 1.61; 95% CI, 1.23–2.11), and postnatal hydrocortisone exposure (OR 2.02; 95% CI 1.50–2.73) were associated with SIP. Infants who lost 15–20% (OR 1.77; 95% CI, 1.28–2.44) or >20% (OR 2.04; 95% CI, 1.46–2.85) of birth weight had higher odds of SIP than infants with weight loss <10%.
Conclusions
Antenatal magnesium exposure, antenatal indomethacin exposure, postnatal hydrocortisone exposure, postnatal indomethacin exposure, and weight loss ≥15% were associated with SIP.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
Code availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author upon reasonable request.
References
Elgendy MM, Othman HF, Heis F, Qattea I, Aly H. Spontaneous intestinal perforation in premature infants: a national study. J Perinatol. 2021;41:1122–8.
Swanson JR, Hair A, Clark RH, Gordon PV. Spontaneous intestinal perforation (SIP) will soon become the most common form of surgical bowel disease in the extremely low birth weight (ELBW) infant. J Perinatol. 2022;42:423–9.
Rysavy MA, Mehler K, Oberthür A, Ågren J, Kusuda S, McNamara N, et al. An immature science: intensive care for infants born at ≤23 weeks of gestation. J Pediatr. 2021;233:16–25.e1.
Gordon PV. Understanding intestinal vulnerability to perforation in the extremely low birth weight infant. Pediatr Res. 2009;65:138–44.
Arnautovic TI, Longo JL, Trail-Burns EJ, Tucker R, Keszler M, Laptook AR. Antenatal risk factors associated with spontaneous intestinal perforation in preterm infants receiving postnatal indomethacin. J Pediatr. 2021;232:59–64.e1.
Kandraju H, Kanungo J, Lee KS, Daspal S, Addie MA, Dorling J, et al. Association of co-exposure of antenatal steroid and prophylactic indomethacin with spontaneous intestinal perforation. J Pediatr. 2021;235:34–41.e1.
Herman AC, Carlisle EM, Paxton JB, Gordon PV. Insulin-like growth factor-I governs submucosal growth and thickness in the newborn mouse ileum. Pediatr Res. 2004;55:507–13.
Gordon PV, Herman AC, Marcinkiewicz M, Gaston BM, Laubach VE, Aschner JL. A neonatal mouse model of intestinal perforation: investigating the harmful synergism between glucocorticoids and indomethacin. J Pediatr Gastroenterol Nutr. 2007;45:509–19.
Rattray BN, Kraus DM, Drinker LR, Goldberg RN, Tanaka DT, Cotten CM. Antenatal magnesium sulfate and spontaneous intestinal perforation in infants less than 25 weeks gestation. J Perinatol. 2014;34:819–22.
Havranek T, Ashmeade TL, Afanador M, Carver JD. Effects of maternal magnesium sulfate administration on intestinal blood flow velocity in preterm neonates. Neonatology. 2011;100:44–49.
Gursoy T, Imamoglu E, Ovali F, Karatekin G. Effects of antenatal magnesium exposure on intestinal blood flow and outcome in preterm neonates. Am J Perinatol. 2015;32:1064–9.
Prasad U, Mohnani A, Hussain N. Spontaneous intestinal perforation associated with premature twin infants. J Neonatal-Perinat Med. 2021;14:403–9.
Suply E, Leclair M-D, Neunlist M, Roze J-C, Flamant C. Spontaneous intestinal perforation and necrotizing enterocolitis: a 16-year retrospective study from a single center. Eur J Pediatr Surg Off J Austrian Assoc Pediatr Surg Al Z Kinderchir. 2015;25:520–5.
Shah TA, Meinzen-Derr J, Gratton T, Steichen J, Donovan EF, Yolton K, et al. Hospital and neurodevelopmental outcomes of extremely low-birth-weight infants with necrotizing enterocolitis and spontaneous intestinal perforation. J Perinatol. 2012;32:552–8.
Spitzer AR, Ellsbury DL, Handler D, Clark RH. The Pediatrix BabySteps® data warehouse and the pediatrix qualitysteps improvement project system—tools for “Meaningful Use” in continuous quality improvement. Clin Perinatol. 2010;37:49–70.
Fenton TR, Kim JH. A systematic review and meta-analysis to revise the Fenton growth chart for preterm infants. BMC Pediatr. 2013;13:59.
Downey LC, Cotten CM, Hornik CP, Laughon MM, Tolia VN, Clark RH, et al. Association of in utero magnesium exposure and spontaneous intestinal perforations in extremely low birth weight infants. J Perinatol. 2017;37:641–4.
Mikhael M, Bronson C, Zhang L, Curran M, Rodriguez H, Bhakta KY. Lack of evidence for time or dose relationship between antenatal magnesium sulfate and intestinal injury in extremely preterm neonates. Neonatology. 2019;115:371–8.
Shalabi M, Mohamed A, Lemyre B, Aziz K, Faucher D, Shah PS, et al. Antenatal exposure to magnesium sulfate and spontaneous intestinal perforation and necrotizing enterocolitis in extremely preterm neonates. Am J Perinatol. 2017;34:1227–33.
Sharma R, Hudak ML, Tepas JJ, Wludyka PS, Teng RJ, Hastings LK, et al. Prenatal or postnatal indomethacin exposure and neonatal gut injury associated with isolated intestinal perforation and necrotizing enterocolitis. J Perinatol. 2010;30:786–93.
Attridge JT, Clark R, Walker MW, Gordon PV. New insights into spontaneous intestinal perforation using a national data set: (1) SIP is associated with early indomethacin exposure. J Perinatol. 2006;26:93–99.
Baud O, Maury L, Lebail F, Duksha R, El Moussawi F, Nicaise C, et al. Effect of early low-dose hydrocortisone on survival without bronchopulmonary dysplasia in extremely preterm infants (PREMILOC): a double-blind, placebo-controlled, multicentre, randomised trial. The Lancet. 2016;387:1827–36.
Rouse DJ, Spong CY, Alexander JM, Malone FD, O’Sullivan MJ, Roberts JM, et al. A randomized, controlled trial of magnesium sulfate for the prevention of cerebral palsy. N Engl J Med. 2008;359:895–905.
Crowther CA, Hiller JE, Doyle LW, Haslam RR. Effect of magnesium sulfate given for neuroprotection before preterm birth: a randomized controlled trial. JAMA. 2003;290:2669–76.
Amin SB, Sinkin RA, Glantz JC. Metaanalysis of the effect of antenatal indomethacin on neonatal outcomes. Am J Obstet Gynecol. 2007;197:486.e1–486.e10.
Gordon PV, Swanson JR, Clark R. Antenatal indomethacin is more likely associated with spontaneous intestinal perforation rather than NEC. Am J Obstet Gynecol. 2008;198:725.
Renolleau C, Toumazi A, Bourmaud A, Benoist J, Chevenne D, Mohamed D, et al. Association between baseline cortisol serum concentrations and the effect of prophylactic hydrocortisone in extremely preterm infants. J Pediatr. 2021;234:65–70.e3.
Shaffer ML, Baud O, Lacaze-Masmonteil T, Peltoniemi OM, Bonsante F, Watterberg KL. Effect of prophylaxis for early adrenal insufficiency using low-dose hydrocortisone in very preterm infants: an individual patient data meta-analysis. J Pediatr. 2019;207:136–142.e5.
Qureshi M, Shah PS, Abdelgadir D, Ye XY, Afifi J, Yuen R, et al. Gestational age-dependent variations in effects of prophylactic indomethacin on brain injury and intestinal injury. J Pediatr. 2021;235:26–33.e2.
Wadhawan R, Oh W, Perritt R, Laptook AR, Poole K, Wright LL, et al. Association between early postnatal weight loss and death or BPD in small and appropriate for gestational age extremely low-birth-weight infants. J Perinatol. 2007;27:359–64.
Starr MC, Griffin R, Gist KM, Segar JL, Raina R, Guillet R, et al. Association of fluid balance with short- and long-term respiratory outcomes in extremely premature neonates: a secondary analysis of a randomized clinical trial. JAMA Netw Open. 2022;5:e2248826.
Zozaya C, Aziz K, Singhal N, Ye XY, Drolet C, Emberley J, et al. Association of weight changes by three days after birth and mortality and/or severe neurological injury in preterm infants <29 weeks gestational age: a multicenter cohort study. Children. 2022;9:276.
Valentine GC, Perez KM, Wood TR, Mayock DE, Comstock BA, Puia-Dumitrescu M, et al. Postnatal maximal weight loss, fluid administration, and outcomes in extremely preterm newborns. J Perinatol. 2022;42:1008–16.
Holland AJA, Shun A, Martin HCO, Cooke-Yarborough C, Holland J. Small bowel perforation in the premature neonate: congenital or acquired? Pediatr Surg Int. 2003;19:489–94.
Sung SI, Ahn SY, Choi S-J, Soo-Young O, Roh C-R, Yang M, et al. Increased risk of meconium-related ileus in extremely premature infants exposed to antenatal magnesium sulfate. Neonatology. 2022;119:68–76.
Funding
This work was funded under the National Institute of Child Health and Human Development (NICHD) contract (HHSN275201 000003I) for the Pediatric Trials Network (PI DKB Jr). This work was also funded by the Biogen Foundation and Duke Clinical Research Institute’s R25 Summer Training in Academic Research (STAR) Program (grant #5R25HD076475-10). The content is solely the authors' responsibility and does not necessarily represent the official views of the National Institutes of Health.
Author information
Authors and Affiliations
Contributions
RK conceptualized and designed the study, supervised the drafting of the manuscript, interpreted the data analyses, and reviewed and revised the manuscript. RK had full access to all the data and takes responsibility for the integrity of the data and the accuracy of the data analysis. PVT contributed to the conception and design of the study, drafted the initial manuscript, and contributed to the data interpretation and reviewing and revising the manuscript. RGG supervised the conception and design of the study, contributed to the data interpretation and the critical revision of the manuscript for important intellectual content. KFS contributed to the data interpretation and the manuscript drafting. C-ABD contributed to the data interpretation and the manuscript drafting. JGH contributed to the data interpretation and the manuscript drafting. JRN contributed to the data interpretation and the manuscript drafting. MP-R contributed to the data interpretation and the manuscript drafting. CMS contributed to the data interpretation and the manuscript drafting. RHC contributed to the data interpretation and the critical revision of the manuscript for important intellectual content. DKB contributed to the data interpretation and the critical revision of the manuscript for important intellectual content. KOZ contributed to the data interpretation and the critical revision of the manuscript for important intellectual content. RNG contributed to the data interpretation and the critical revision of the manuscript for important intellectual content. NY contributed to the data interpretation and the critical revision of the manuscript for important intellectual content. DT contributed to the data interpretation and the critical revision of the manuscript for important intellectual content. PBS contributed to the design of the study, data interpretation, and the critical revision of the manuscript for important intellectual content. All authors approved the final manuscript as submitted and agree to be accountable for all aspects of the work.
Corresponding author
Ethics declarations
Competing interests
PT, C-AD, JGH, JN, MP-R, and CS declare support from a National Institute of Child Health and Human Development grant. DKB reports consultancy for Allergan, Melinta Therapeutics, Sun Pharma Advanced Research Co. KOZ reports funding from the National Institutes of Health (NIH) and US Food and Drug Administration (FDA). RGG has received support from the industry for research services (https://dcri.org/about-us/conflict-of-interest/). KFS, RNG, NY, DT, PBS, RC, and RK have nothing to disclose.
Ethics approval
The Duke University Institutional Review Board (Durham, NC USA) provided permission to conduct this analysis (Protocol ID: Pro00111546)
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
High school or college students (Pavan V. Thakkar, Chloe-Ann B. Detwiler, Julia G. Henegar, Jai R. Narayan, Melanie Perez-Romero, and Ciara M. Strausser are affiliated with the Duke Clinical Research Institute’s R25 Summer Training in Academic Research (STAR) Program.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Thakkar, P.V., Sutton, K.F., Detwiler, CA.B. et al. Risk factors and epidemiology of spontaneous intestinal perforation among infants born at 22–24 weeks’ gestational age. J Perinatol 44, 94–99 (2024). https://doi.org/10.1038/s41372-023-01782-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41372-023-01782-6
This article is cited by
-
Neonatal Necrotizing Enterocolitis: An Update on Pathophysiology, Treatment, and Prevention
Pediatric Drugs (2024)