Abstract
Objective
To identify bacteria in umbilical cord tissue and investigate the association with placental inflammation and neonatal sepsis risk score.
Study design
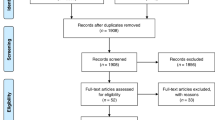
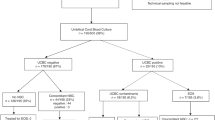
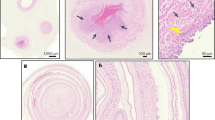
Retrospective cohort study from 2017–2019. RNA was extracted from umbilical cord tissue and NanoString nCounter used to identify seven bacteria genera. Sepsis risk score was calculated using the Kaiser sepsis calculator. Placental histopathology was abstracted from medical records.
Results
Detection of bacterial RNA in the umbilical cord (n = 96/287) was associated with high-stage maternal and fetal acute placental inflammation (maternal 35.4% vs 22.5%, p = 0.03 and fetal 34.4% vs 19.4%, p < 0.01) and maternal vascular malperfusion (36.5% vs 23.0%, p = 0.02). Detection of Ureaplasma spp. was also associated with increased sepsis risk score (1.5/1000 [0.6, 8.6] vs 0.9/1000 [0.2, 2.9], p = 0.04).
Conclusion
Umbilical cord bacterial pathogens are linked to fetal and maternal placental inflammation and maternal vascular malperfusion during gestation and associated with increased sepsis risk score in the neonate.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others
Data availability
The datasets generated during and/or analyzed during the current study are not publicly available due potential compromise of individual privacy, but are available from the corresponding author on reasonable request.
References
Simonsen KA, Anderson-Berry AL, Delair SF, Davies HD. Early-onset neonatal sepsis. Clin Microbiol Rev. 2014;27:21–47.
Cai S, Thompson DK, Anderson PJ, Yang JY. Short- and long-term neurodevelopmental outcomes of very preterm infants with neonatal sepsis: a systematic review and meta-analysis. Children. 2019;6:131.
Klingenberg C, Kornelisse RF, Buonocore G, Maier RF, Stocker M. Culture-negative early-onset neonatal sepsis - at the crossroad between efficient sepsis care and antimicrobial stewardship. Front Pediatr. 2018;6:285.
Kuppala VS, Meinzen-Derr J, Morrow AL, Schibler KR. Prolonged initial empirical antibiotic treatment is associated with adverse outcomes in premature infants. J Pediatr. 2011;159:720–5.
Zhu K, Gao H, Yuan L, Wang L, Deng F. Prolonged antibiotic therapy increased necrotizing enterocolitis in very low birth weight infants without culture-proven sepsis. Front Pediatr. 2022;10:949830.
Zou Z, Liu W, Huang C, Sun C, Zhang J. First-year antibiotics exposure in relation to childhood asthma, allergies, and airway illnesses. Int J Environ Res Public Health. 2020;17:5700.
Leong KSW, McLay J, Derraik JGB, Gibb S, Shackleton N, Taylor RW, et al. Associations of prenatal and childhood antibiotic exposure with obesity at age 4 years. JAMA Netw Open. 2020;3:e1919681.
Olomu IN, Hecht JL, Onderdonk AO, Allred EN, Leviton A. Extremely low gestational age newborn study I. Perinatal correlates of ureaplasma urealyticum in placenta parenchyma of singleton pregnancies that end before 28 weeks of gestation. Pediatrics. 2009;123:1329–36.
Fichorova RN, Onderdonk AB, Yamamoto H, Delaney ML, DuBois AM, Allred E, et al. Maternal microbe-specific modulation of inflammatory response in extremely low-gestational-age newborns. mBio. 2011;2:e00280–10.
Leviton A, Hecht JL, Allred EN, Yamamoto H, Fichorova RN, Dammann O, et al. Persistence after birth of systemic inflammation associated with umbilical cord inflammation. J Reprod Immunol. 2011;90:235–43.
Kuzniewicz MW, Puopolo KM, Fischer A, Walsh EM, Li S, Newman TB, et al. A quantitative, risk-based approach to the management of neonatal early-onset sepsis. JAMA Pediatr. 2017;171:365–71.
Puopolo KM, Draper D, Wi S, Newman TB, Zupancic J, Lieberman E, et al. Estimating the probability of neonatal early-onset infection on the basis of maternal risk factors. Pediatrics. 2011;128:e1155–63.
Kuzniewicz MW, Walsh EM, Li S, Fischer A, Escobar GJ. Development and implementation of an early-onset sepsis calculator to guide antibiotic management in late preterm and term neonates. Jt Comm J Qual Patient Saf. 2016;42:232–9.
NanoString Technologies I. nSolver 4.0 analysis software user manual. Seattle, Washington 2018. Available from: https://nanostring.com/wp-content/uploads/MAN-C0019-08_nSolver_4.0_Analysis_Software_User_Manual.pdf.
Team RC. R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria, 2021.
Budal EB, Bentsen MHL, Kessler J, Ebbing C, Lindemann PC, Haugen OH, et al. Histologic chorioamnionitis in extremely preterm births, microbiological findings and infant outcome. J Matern Fetal Neonatal Med. 2023;36:2196599.
Stol K, Jans J, Ott de Bruin L, Unger W, van Rossum A. Perinatal infections with ureaplasma. Pediatr Infect Dis J. 2021;40:S26–30.
Sweeney EL, Kallapur SG, Meawad S, Gisslen T, Stephenson SA, Jobe AH, et al. Ureaplasma species Multiple Banded Antigen (MBA) variation is associated with the severity of inflammation in vivo and in vitro in human placentae. Front Cell Infect Microbiol. 2017;7:123.
Bartkeviciene D, Opolskiene G, Bartkeviciute A, Arlauskiene A, Lauzikiene D, Zakareviciene J, et al. The impact of Ureaplasma infections on pregnancy complications. Libyan J Med. 2020;15:1812821.
Xu YP, Hu JM, Huang YQ, Shi LP. Maternal Ureaplasma exposure during pregnancy and the risk of preterm birth and BPD: a meta-analysis. Arch Gynecol Obstet. 2022;306:1863–72.
Kallapur SG, Kramer BW, Jobe AH. Ureaplasma and BPD. Semin Perinatol. 2013;37:94–101.
Kasper DC, Mechtler TP, Reischer GH, Witt A, Langgartner M, Pollak A, et al. The bacterial load of Ureaplasma parvum in amniotic fluid is correlated with an increased intrauterine inflammatory response. Diagn Microbiol Infect Dis. 2010;67:117–21.
Motomura K, Hara M, Ito I, Morita H, Matsumoto K. Roles of human trophoblasts’ pattern recognition receptors in host defense and pregnancy complications. J Reprod Immunol. 2023;156:103811.
Ernst LM. Maternal vascular malperfusion of the placental bed. APMIS. 2018;126:551–60.
Mor G, Kwon JY. Trophoblast-microbiome interaction: a new paradigm on immune regulation. Am J Obstet Gynecol. 2015;213:S131–7.
Ben Amara A, Gorvel L, Baulan K, Derain-Court J, Buffat C, Verollet C, et al. Placental macrophages are impaired in chorioamnionitis, an infectious pathology of the placenta. J Immunol. 2013;191:5501–14.
Barron A, McCarthy CM, O’Keeffe GW. Preeclampsia and neurodevelopmental outcomes: potential pathogenic roles for inflammation and oxidative stress? Mol Neurobiol. 2021;58:2734–56.
Freedman AA, Keenan-Devlin LS, Borders A, Miller GE, Ernst LM. Formulating a meaningful and comprehensive placental phenotypic classification. Pediatr Dev Pathol. 2021;24:337–50.
Funding
This study was funded by the Auxiliary of NorthShore University HealthSystem (PI: Ernst).
Author information
Authors and Affiliations
Contributions
ADF assisted with data analysis and interpretation, gathered clinical data, drafted the initial manuscript, and reviewed and revised the manuscript. AF performed statistical analysis and reviewed and revised the manuscript. KW assisted with data analysis and interpretation and reviewed and revised the manuscript. KAM oversaw the RNA extraction and NanoString analysis, interpreted data, and reviewed and revised the manuscript. VW performed the sectioning and RNA extractions from the umbilical cord tissue and reviewed and revised the manuscript. EP assisted with patient selection, data collection, maintained the integrity of the study database and reviewed and revised the manuscript. LME conceptualized the study design, interpreted data, reviewed the placental histology, and reviewed and revised the manuscript. All authors approve the final manuscript as submitted and agree to be accountable for all aspects of the work.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Franklin, A.D., Freedman, A., Wylie, K. et al. Molecular detection of bacteria, placental inflammation, and neonatal sepsis risk. J Perinatol 44, 46–54 (2024). https://doi.org/10.1038/s41372-023-01775-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41372-023-01775-5