Abstract
To inform changes to the Supporting and Enhancing NICU Sensory Experiences (SENSE) program, studies investigating sensory-based interventions in the NICU with preterm infants born ≤32 weeks were identified. Studies published between October 2015 to December 2020, and with outcomes related to infant development or parent well-being, were included in this integrative review. The systematic search used databases including MEDLINE, Cumulative Index to Nursing and Allied Health Literature, the Cochrane Library, and Google Scholar. Fifty-seven articles (15 tactile, 9 auditory, 5 visual, 1 gustatory/olfactory, 5 kinesthetic, and 22 multimodal) were identified. The majority of the sensory interventions that were identified within the articles were reported in a previous integrative review (1995–2015) and already included in the SENSE program. New evidence has led to refinements of the SENSE program, notably the addition of position changes across postmenstrual age (PMA) and visual tracking starting at 34 weeks PMA.
Similar content being viewed by others
Background
The neonatal intensive care unit environment (NICU) has been described as noisy and chaotic, and high-risk infants in the NICU are often exposed to invasive and painful medical interventions [1] and lack positive and consistent forms of sensory exposures [2]. The provision of consistent forms of positive and appropriately-timed sensory exposures to high-risk infants in the NICU can improve the safety and quality of care in the NICU. This is important, as very preterm infants require care in the NICU for an average of 3 months after birth [3], and the adverse NICU environment can have negative effects on early brain structure and function [1, 3]. Early brain development is susceptible to external stimuli [4, 5], and this modifiable factor could improve not only the NICU experience for parents and infants, but lead to better infant and parent outcomes.
In 2015, an integrative review was conducted to identify positive sensory exposures for high-risk infants in the NICU. The review identified 88 articles [6] with evidence over the previous 20 years (1995–2015). A large portion of literature supported the use of kangaroo care (also termed skin-to-skin), music and language exposure, and multimodal interventions starting at 25–28 weeks postmenstrual age (PMA). Such interventions have evidence supporting improvements in infant development, sleep, and physiology, as well as lower maternal stress. Although evidence exists that sensory interventions (kangaroo care, massage, music) relate to better parent and infant outcomes [7,8,9,10], most interventions for preterm infants in the NICU were implemented inconsistently and/or for short periods of time across studies [6], reducing their impact.
The initial integrative review was the first step in the development of the Supporting and Enhancing NICU Sensory Experiences (SENSE) program. The SENSE program includes specific doses and targeted timing of interventions such as massage, auditory exposure, rocking, holding, and kangaroo care [11]. The goal is to empower parents to engage in the described sensory activities with their infant(s). In addition to the initial integrative review [6], a rigorous process of protocol development for the SENSE program took place, which included expert input from a multidisciplinary group of 108 health care professionals that defined sensory interventions implemented across different NICUs, 3 multidisciplinary focus groups that provided a critical review of the guideline [12, 13], and interviews with 20 mothers of preterm infants who gave input on feasibility of implementing the SENSE program in the NICU [14]. All of these results were integrated to develop the evidence-based SENSE program.
Since then, a pilot study of 30 preterm infants who received the SENSE program compared to 50 historical controls demonstrated the feasibility of implementing the SENSE program in a level IV NICU and preliminary evidence of a positive impact on parent confidence and infant neurobehavior [15]. A randomized clinical trial of 70 parent-infant dyads identified more lethargy among infants who received the SENSE program, even after controlling for medical and social factors [16]. Better language outcomes on the Ages and Stages Questionnaire at 1 year of age were observed in the infants who received the SENSE program, but this was no longer significant after controlling for medical and social factors. Implementation research has identified that the SENSE program can be adopted with good fidelity [17]. An additional study examining the impact of the SENSE program on infants (n = 110), parents, and health care professionals reported that NICU personnel identified that having bedside information on appropriate sensory exposures (included in the SENSE program) enhanced their ability to deliver care and information to the caregiver [18]. Parents who received the SENSE program reported feeling better prepared for the transition to home and very satisfied with the quality of care they received in the NICU. Improved feeding outcomes in the group that received the SENSE program were also reported, with infants in the SENSE group demonstrating a decrease in the number of days between first gavage feeding and full oral feeding.
The SENSE program was made available to other hospitals in June of 2018, and more than 400 hospitals around the world have obtained the SENSE program. The cost of the SENSE program is used to support distribution, with no direct financial benefit to an entity or individual. NICU practice continues to evolve, as does the evidence that supports NICU care. Therefore, it is important to update the SENSE program as new evidence becomes available. Here, we report on an updated integrative review of evidence from 2015–2020 related to sensory-based interventions in the NICU to inform changes made to the SENSE program in 2022.
Methods
Purpose
The purpose of this review is to report evidence from October 2015–December 2020 related to sensory-based interventions in the NICU associated with positive infant and parent outcomes, in order to inform refinements to the SENSE program.
Procedures
An integrative review was used to highlight the most relevant evidence related to sensory exposures in the NICU from a range of clinical research methodologies. Various study designs (systematic reviews, randomized controlled trials, quasi-experimental with subjects assigned to groups without use of randomization, crossover, or single-group repeated measure studies) published over a 5-year period (2015–2020) were considered for inclusion. The population of interest was preterm infants born ≤32 weeks gestation who had a sensory-based intervention that commenced prior to 36 weeks PMA while in the NICU. Studies that imposed a quantifiable environmental sensory exposure (tactile, auditory, vestibular, kinesthetic, visual, olfactory/gustatory, or multimodal) during the NICU stay were included. The comparison group received either (1) no identified sensory intervention, (2) standard of care, (3) varying levels of the same or similar intervention, or (4) a different sensory exposure. Interventions could be performed by healthcare workers, research team members, or parents. Relevant outcomes included infant behavioral outcomes (such as fewer observations of stress), neurobehavioral outcomes (including higher scores on standardized neurobehavioral or neurodevelopmental testing), parent well-being (less reported stress, anxiety, depression on standardized measures), and other parental outcomes (better confidence, more reports of engagement with infant in the NICU). Studies with outcomes related solely to pain or feeding were excluded, as these complex constructs deserve their own investigation and programming. Samples of healthy infants were excluded, as this review intended to define sensory exposures for medically complex preterm infants in the NICU. Studies with sample size <30 and without an a priori calculation of power that was met were excluded. Due to their inclusion in the previous integrative review (and related to the heterogeneity of outcome measures across studies), we included outcomes of bone health, growth, gastrointestinal function, lab values, infant physiology (fewer desaturation events, heart rate, respiratory rate), and length of stay in this review. However, we have denoted those studies as ones that will not inform refinements to the SENSE program, because they are a departure from the primary outcome of interest. The primary outcome of interest was infant neurobehavior or neurodevelopment.
See Table 1 for the exclusion criteria for this review. See Table 2 for search criteria and keywords.
Search strategy
A systematic search for studies published from October 2015 to October 2020 was performed using databases including MEDLINE (via PubMed), CINAHL (Cumulative Index to Nursing and Allied Health Literature), the Cochrane Library, and Google Scholar. Reference lists of included studies were also searched for relevant literature. Searches were performed separately for each sensory topic (tactile, auditory, visual, kinesthetic, vestibular, olfactory/gustatory, and multimodal). A combination of search terms was used, including those focused on preterm infants and the sensory exposure(s) of interest.
Study screening
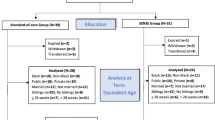
Two reviewers set the search engine and screened articles for inclusion. One reviewer (AG or RG) first screened studies for inclusion by title. In situations where the title was unclear, the abstract was retrieved for review. The full text articles of potentially relevant studies were reviewed for final inclusion by both reviewers. If relevance of an intervention or inclusion of a study was unclear after review by both reviewers, it was resolved through discussion with the review team (RP, AG, RG, PK, and JS). See Fig. 1 for the flow diagram defining the total number of articles reviewed and how many remained after exclusions.
Data extraction
One reviewer performed data extraction that was checked for accuracy by a second reviewer (AG or RG). Extracted information included study design, sample size, country of origin, intervention (including frequency, duration, timing), estimated gestational age (EGA) at birth, PMA at intervention, study inclusion/exclusion criteria, and study outcomes and results. When results from the same sample were reported in multiple publications, they were reported together in this review as a single study. When it was unclear if samples came from the same cohort, authors were contacted for confirmation.
Study quality
Assessment of study quality was independently performed by two reviewers (AG and RG), and disagreements regarding study quality were resolved by discussion among the two reviewers until consensus was achieved. Systematic reviews were assessed for methodological quality using the Documentation and Appraisal Review Tool (DART) [19]. The remaining studies were assessed for quality using a modified version of a tool developed by the United Kingdom’s National Institute for Health and Care Excellence (NICE [20]). The tool evaluates studies for selection bias (randomization, allocation concealment, group comparability at baseline), performance bias (groups received the same care, blinding of participants and healthcare workers), attrition bias (equal follow-up time, completion of treatment, complete outcome data), detection bias (appropriate length of follow-up, precise definition of outcomes, valid and reliable outcomes, blinding of investigators or outcome assessor), and other bias (statistical methods, issues related to specific study designs). Each factor was rated as yes/adequate, no/inadequate, or unclear. Several of these factors were not relevant for single-group repeated measures studies (Appendix B).
Results
See Fig. 1 for a breakdown of the articles reviewed during the integrative review process. See Fig. 2 for evidence of the different types of interventions across PMA. See Appendix A for the 49 cohorts (and 57 published articles) included in this review (13 cohorts on tactile across 15 publications, 6 cohorts on auditory across 9 publications, 3 cohorts on kinesthetic across 5 publications, 1 cohort on olfactory/gustatory across 1 publication, 5 cohorts on visual across 5 publications, and 21 cohorts on multimodal interventions across 22 publications).
Synthesis of findings
Given the significant heterogeneity of studies and their outcomes, study findings could not be combined quantitatively, but were summarized qualitatively. Evidence related to each type of sensory intervention was defined across each PMA, to determine at what age of maturity evidence existed to support specific interventions (Fig. 2). Further, all outcomes were reported for each sensory exposure, but outcomes related to neurobehavior or neurodevelopmental outcomes were highlighted. See Appendix C for interventions across PMA from 1995–2020, inclusive of evidence from previous and current integrative reviews.
Tactile
The previous 1995–2015 review identified evidence-based tactile exposures to be gentle human touch [21,22,23,24], massage [25,26,27], and kangaroo care or skin-to-skin [7, 28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49]. This new 2015–2020 integrative review added 15 articles (13 cohorts) all of which described kangaroo care being done for 15 min [50] to 2 h [51, 52] starting as early as 24 weeks PMA [53]. Two studies evaluated kangaroo care specifically in the delivery room immediately after birth, starting at 25 weeks PMA [54, 55]. The frequency of kangaroo care across studies varied and ranged from 1 time per day [50] to an average of 4 times per day [56, 57]. The length of time that kangaroo care was studied ranged from 1 day [53, 58,59,60] to 3 weeks [50]. No new tactile interventions were identified since the previous review. However, multimodal interventions often included a tactile component (see “Multimodal” section below). Positive outcomes associated with increased duration and/or frequency of kangaroo care included stabilized respiratory rate [58, 61], improved maternal-infant attachment [54, 55, 61, 62], decreased parental stress [50, 54, 55, 61, 63], lower heart rate [58, 60], increased short-term electromyographic activity of the biceps brachii and hamstrings [59], increased oxygen saturation [51], decreased infant salivary cortisol levels [64], increased salivary oxytocin levels for infants and parents [64], decreased anxiety for parents [64], decreased risk of early postpartum depression or impaired bonding [54, 55], increased weight gain, fewer episodes of apnea, decreased use of formula, improved sleep, and decreased crying [52]. One study related a tactile exposure to the primary outcome of interest (neurobehavioral or neurodevelopmental outcome): longer duration of kangaroo care (120 versus 60 min over 7 days) starting at 31 weeks PMA was related to better scores for attention, arousal, regulation, non-optimal reflexes, quality of movement, handling, excitability, and lethargy on the NICU Network Neurobehavioral Scale [51].
Auditory
The previous 1995–2015 review identified evidence-based auditory exposures to be live music/singing [65, 66], recorded music/singing/maternal voice [67,68,69,70,71], and recorded maternal biological sounds [72, 73]. This new 2015–2020 integrative review added an additional 9 articles, representing 6 different cohorts, on auditory interventions. Auditory interventions described included recorded music or voice and live music or voice. Auditory interventions started as early as 25 weeks PMA [74] and lasted from 8 min [75,76,77,78] to 30 min 5x/day for a total of 150 min per day [74]. Studies occurred for as short as 1 day [79, 80] and as long as 6–10 weeks [74]. Some studies used headphones, and others did not. Live musical instruments used included the pentatonic harp [80]. Recordings included Brahm’s lullaby, punji, bells, harp, and voice (folkloric lullaby, humming, singing, reading, talking). Auditory interventions were also described within other bundled multisensory programs [15, 81]. Not all studies reported decibel levels; those that did reported ranges between 65–75 decibels [74, 82], while some packaged programs, such as the SENSE program, described auditory interventions at the American Academy of Pediatrics’ recommendation of ≤45 decibels [83]. One study identified use of an auditory stimulus played 5–15 decibels above background noise [79]. Studies over the past 5 years identified positive outcomes associated with recorded maternal lullaby from 28–34 weeks PMA, recorded music from 33–40 weeks PMA, recorded female voice from 25–40 weeks PMA, and live harp music from 33–37 weeks PMA. In the multimodal category, additional studies were identified that combined auditory stimulation (namely maternal voice and music) with kangaroo care, therapeutic touch, and massage. An auditory component (voice/singing with daily dose recommendations based on infant PMA) was also included as part of the packaged SENSE program [15]. Outcomes related to recorded music starting at 33 weeks PMA included increased brain connectivity [76, 78] and improved white matter maturation and larger amygdala volumes [77]. No differences in neurodevelopmental outcome were noted in groups that received recorded music as an intervention [75, 79]. Recorded maternal voice starting as early as 28 weeks (talking, reading, and/or singing) was related to parents having less fear, discontent with baby, burden, and increased rates of breastfeeding as well as increased infant oxygenation and decreased heart rate [84]. One study related auditory exposures (30 min of recorded maternal voice, 5 times per day for a total of 150 min per day) starting 2 weeks after birth (as early as 25 weeks PMA) over 6–10 weeks to the primary outcome of interest: recorded maternal voice was related to improved neurodevelopmental outcome at 5 months on the Griffith Scales (but not at 20 months) [74].
Visual
The previous 1995–2015 review identified cycled light as the only evidence-based visual intervention [85,86,87]. This new 2015–2020 integrative review added an additional 5 articles, all of which examined cycled light with an attempt to better pinpoint reactions to light as well as appropriate timing of initiation of cycled light. In these studies, cycled lighting was introduced as early as 28 weeks PMA [88, 89], however, the comparator was starting cycling at 36 weeks which did not enable pinpointing whether timing of cycling at 28 weeks compared to 32 weeks PMA (when cycling is introduced in most instances) differed. Lux levels ranged from 1–30 lux at night and 40–600 lux during the day, with most falling around 200–250 lux during the day. There was also a new Cochrane review of cycled light published in 2017 [90], but all of the articles described were already captured in our previous integrative review. Four articles with visual components were included in the multimodal category as visual stimulation was a component of other sensory programs or experiences, either via cycled lighting [15], with eye contact during music therapy (20-min sessions, 2–3x/week) [81], with a darkened environment using a cover over the incubator until initiation of oral feeding [91], or with visual tracking [92]. Visual tracking of a parent’s face or toy was described starting at 34 weeks PMA 6 times per week with positive outcomes related to improved visual performance [92]. Further, one study investigated the use of red light, but there were no significant outcomes related to its use [93]. There were no studies that related visual exposures alone to the primary outcome of interest.
Kinesthetic
The previous 1995–2015 review identified evidence-based kinesthetic interventions to be physical therapy [94, 95] with most outcomes related to bone health. This new 2015–2020 integrative review added an additional 5 articles, representing 3 cohorts. Most reported on passive range of motion (ROM) with bone-related outcomes. None of the studies on passive ROM alone demonstrated significant differences in the outcomes of interest. Movement exercises started as early as 28 weeks PMA [96] and consisted of ~7–10 min of exercise each day, with some occurring 2 times per day. Three articles (all reporting on the same cohort) assessed guided movement which consisted of different positions to improve postural control, head control, and midline orientation. This was done for 10 min twice per day at 34, 35, and 36 weeks PMA, with positive outcomes on the Test of Infant Motor Performance (TIMP) identified [97,98,99]. One study with a kinesthetic component was categorized as multimodal, with passive ROM coupled with gentle joint compression during kangaroo care between 29–37 weeks PMA, with positive outcomes identified and listed in the multimodal section below [51]. Another multimodal sensory program, the SENSE program, included a 2-min movement opportunity up to 1–8 times per day, increasing in frequency dependent upon the infant’s PMA and tolerance [15]. Six studies, categorized as multimodal, used the Field protocol for infant massage, which consists of 5 min of tactile input, 5 min of vestibular input, and an additional 5 min of tactile input for a total of 15 min of intervention [100,101,102,103,104,105]. One of these studies found positive outcomes, including increased weight gain, associated with conducting Field massage three times per day for 15 consecutive days, between 30–35 weeks PMA, with the infant in kangaroo care [100]. A similar protocol consisted of a 15-min massage with tactile and vestibular components and was implemented 2 times per day for 14 days between 30–36 weeks PMA [106]. Although not directly related to the outcomes of interest, daily range of motion exercises were associated with higher tibial speed of sound measures as an indicator of bone mineral density and specifically osteopenia [96]. Two studies (in one cohort) found kinesthetic interventions to be related to the primary outcome of interest: parent-administered guided movement starting at 34 weeks PMA (10 min twice per day for 3 weeks) to improve postural control, head control, and midline orientation was associated with higher z-scores on the TIMP at 37 weeks PMA [98, 99].
Gustatory and olfactory
The previous 1995–2015 review identified evidence-based olfactory/gustatory interventions as oropharyngeal colostrum, breast milk odor, or mother’s scent [107,108,109]. This new 2015–2020 integrative review added one additional article on gustatory/olfactory interventions which described a parent scented positioning device which was used between 30–36 weeks PMA for at least 12 h at a time [110]. Another study in the multimodal category compared maternal voice to breast milk odor to incubator cover (darkened environment) to standard of care and found that the breast milk odor group had the shortest duration to full oral feeding [91]. Infants in the breast milk odor group were exposed one time per day for 3 h at a time to 5 ml of breast milk poured into a sterile sponge and positioned 5 centimeters from the infant between 30–32 weeks PMA. Additionally, a study in the multimodal category looked at massage with coconut oil, which reportedly has a subtle odor [111]. Positive outcomes were identified for infants who received massage with 5 ml of coconut oil three times per day between 30–40 weeks PMA and included higher mean weight gain, less hypothermia and apnea, better Neonatal Skin Condition Scores, and better scores on the Developmental Assessment Scale for Indian Infants at 3, 6, and 12 months. There were no studies that related unimodal olfactory/gustatory exposures to the primary outcome of interest.
Multimodal
The previous 1995–2015 review identified evidence-based multimodal interventions as Auditory, Tactile, Visual, and Vestibular (ATVV), Family Nurture Intervention, Hospital to Home Transition-Optimizing Premature Infant’s Environment (H-Hope), massage with aromatic oil, kangaroo care coupled with auditory exposure, and massage intervention with a kinesthetic component [8, 106, 112,113,114,115,116,117,118,119,120,121,122,123,124,125,126,127,128,129,130,131,132,133,134,135,136,137,138,139,140,141,142,143,144,145,146]. This new 2015–2020 integrative review added an additional 22 articles (representing 21 cohorts) on multimodal interventions. Multimodal interventions described included the SENSE program, the Fontana multisensory program, kangaroo care coupled with an auditory intervention, massage (with a kinesthetic component), massage coupled with olfactory input, massage coupled with the Premature Infant Oral Motor Intervention (PIOMI), therapeutic static touch coupled with auditory input, kangaroo care coupled with passive ROM, and ATVV. Tactile and kinesthetic stimulation was related to increased neuromuscular maturity [101], improved sleep [102], increased weight and length [103, 104], increased head circumference [104], and decreased heart rate [103]. Kangaroo care and kinesthetic stimulation was related to improved weight gain [100]. Kangaroo care with music was related to improved mother-infant attachment [147], decreased maternal anxiety [148], and improved weight gain [148]. Massage was associated with increased length, larger chest circumference, increased number of bowel movements, and decreased frequency of pre-feed gastric residual [106]. Massage with oil was associated with decreased weight loss, decreased incidence of hypothermia, decreased incidence of apnea, better skin condition, and decreased length of hospitalization [105, 111]. Touch and auditory stimulation were associated with improved sleep and more stable oxygenation [149]. ROM with compression was associated with higher serum phosphorus levels, lower levels of urinary calcium/phosphate, higher bone mineral density, increased weight gain, and lower alkaline phosphate levels [51]. In relation to the outcomes of interest, bundled multimodal sensory interventions were associated with improved visual reception, improved early learning composite scores, and improved infant neurobehavior [15, 81] as well as improved maternal confidence [15]. Massage with coconut oil was related to improved motor and mental developmental quotients on the Development Assessment Scale for Indian Infants [111], and PIOMI with massage was related to improved neurodevelopmental outcomes on the Ages and Stages Questionnaire [150].
Vestibular
The previous and new reviews added no new studies pertaining to isolated vestibular interventions, though several vestibular interventions were coupled with other sensory interventions as noted in the other sections.
Discussion
The key findings of this review are that (1) there is additional evidence that further supports previously reported sensory-based interventions, (2) there are a few additional sensory-based interventions described in the literature not reported in the previous integrative review, (3) there continue to be significant differences in the reporting of sensory exposure outcomes, dosages, and timing, making it challenging to combine studies or have a cohesive understanding of sensory exposures across PMA, and (4) many studies fail to clearly identify the PMA at which the interventions were conducted. Understanding evidence that emerged from 2015–2020 has led to refinements to the SENSE program. The evidence from 2015–2020 informed changes to the SENSE-II program, including the addition of kinesthetic interventions (position changes) and the use of visual tracking.
Kangaroo care, or skin-to-skin care, appears to have the strongest evidence among sensory-based interventions in the NICU setting. Additional support of kangaroo care, including the use of it starting in the delivery room, emerged in this new review. Further, auditory interventions were defined with one study estimated as starting as early as 25 weeks PMA [74]. Consistent with previous reports, the use of live music or recorded music is not well-reported until at least 32 weeks PMA. Evidence to support timing of cycled light prior to 32 weeks was not isolated, however, evidence to avoid cycled lighting during earlier PMA also does not exist. There continues to be significant variability in cycled light protocols, with variation in lux levels used during day/night cycles. Further, the use of visual tracking of a parent face or toy starting at 34 weeks PMA was a new addition to the evidence [92], as previously there was very little to support visual interventions except within the context of the light environment. Guided movement in different positions was also a new intervention described in the literature starting at 34 weeks PMA [97,98,99]. Evidence on olfactory interventions continue to support parent smell and breast milk with some description of multimodal interventions that included the smell of coconut oil during massage. Multiple new bundled interventions have also emerged in the literature.
There remains little evidence investigating long-term outcomes from sensory interventions. Kangaroo care was related to less infant distress, more comfort, better attachment, improved parent mental health, improved infant sleep, decreased infant crying, and improved infant neurobehavior at term [50,51,52, 54,55,56,57,58, 61,62,63,64]. Auditory interventions were related to brain structure on MRI, development at 5 months of age, as well as personal-social, emotional stability, and language outcomes [75,76,77,78]. Guided movement related to improvements on neurobehavioral outcome measures at 3 months of age [99]. Multimodal interventions also were related to long-term neurodevelopmental outcomes [111, 150]. However, a large amount of the current literature remains focused on shorter term outcomes that have relevance that is questionable for the purposes of this review. An absence of evidence does not mean these interventions do not improve long-term outcomes. Rather, it is a call for research to better understand the long-term impact of sensory-based interventions in the NICU.
It is important to note that research of interventions in a tightly controlled study is different from implementation of interventions in the real world, with the latter often times lacking highly skilled personnel, being done during other concurrent interventions, and without use of strict exclusion criteria [151]. Careful consideration as to whether each intervention can be done for most infants at a given PMA is complex, and vulnerability of infants in the real-world context must be carefully evaluated. Subsequently, adaptations may be needed [152] in order to adequately document the effects of interventions following implementation.
Limitations of included studies
There is a possibility of publication bias, where only studies reporting positive outcomes were published and included in this review. In addition, most studies included multiple outcome measures, many of which did not reach significance. We reported studies that were included in this review in Fig. 2, color coded depending on if the articles had at least one positive outcome that reached significance; however, studies with multiple outcomes (especially those with findings that did not reach significance) may not be well-represented in the PMA tables. We included multiple research designs in an effort to capture all appropriate literature related to improving the sensory environment, and it is possible that lower quality, non-randomized designs could have biased or decreased the strength of the review findings. Of the studies that were randomized, many did not specify methods clearly or report allocation concealment. These, in addition to incomplete or weak assessments of participants at baseline, placed many of these studies at high-risk for selection bias. While participants could not be blinded, and it may be difficult to blind parents and healthcare workers to the intervention, few studies attempted to blind the outcomes assessor. Completeness of treatment and follow-up was also difficult to ascertain, as studies infrequently reported the number of infants by group with complete outcomes data and reasons for loss to follow-up. Most interventions were short in duration and were not conducted across the majority of hospitalization, which limits the strength and generalizability of findings. Finally, generalizability is limited due to the integration of multiple different studies conducted on different populations and in different environments.
Limitations of this review
This review was limited by its focus on parent outcomes and infant neurodevelopmental outcomes. Therefore, evidence with outcomes related to pain, breastfeeding success, nutritional/growth, feeding outcomes, and other important clinical markers may have been excluded. Some studies that reported on the aforementioned outcomes are reported in this review when multiple outcomes were reported, including the outcomes of interest. This review did not include studies printed in languages other than English and did not include non-published literature. Only 1 reviewer screened studies and performed initial data extraction. Exclusion of studies with a sample size less than 30 may have excluded relevant literature. The size and scope of this review also did not allow us to comprehensively, consistently, and repeatedly follow-up with individual study authors in situations where methods or data were missing or unclear. In addition, this review is limited by lack of common interventions and outcomes, as well as failure to elucidate the PMA at which interventions were administered, making it difficult to combine results into a cohesive whole. Because of the significant heterogeneity of the studies included, interpretation is largely qualitative.
Conclusion
In conclusion, this review informed changes to the SENSE program. These additions included refinements of language, expansion of acceptable light protocols prior to 32 weeks PMA, and the addition of position changes, movement, and visual tracking activities. While this new evidence from 2015–2020 informed proposed changes to the SENSE program, suggested changes were then vetted through an expert advisory team. Since the basis of the SENSE program is already established, and research on its efficacy is ongoing, future reviews that will take place every 5 years may focus on randomized clinical trials as the basis for change and only include studies that clearly define the timing (PMA) of the intervention and have outcomes related to the primary outcome of interest, neurobehavioral or neurodevelopmental outcome. Using such a systematic approach of study design inclusion will decrease the challenges associated with inclusion of studies with different study quality and enable the value to be placed on rigorous study designs to inform further change to the SENSE program. Committing to a review of new studies every 5 years will ensure the SENSE program remains updated, based on current evidence.
Data availability
Data available within the article or its Supplementary Materials.
References
Smith GC, Gutovich J, Smyser C, Pineda R, Newnham C, Tjoeng TH, et al. Neonatal intensive care unit stress is associated with brain development in preterm infants. Ann Neurol. 2011;70:541–9.
Lickliter R. The integrated development of sensory organization. Clin Perinatol. 2011;38:591–603.
Pineda RG, Neil J, Dierker D, Smyser CD, Wallendorf M, Kidokoro H, et al. Alterations in brain structure and neurodevelopmental outcome in preterm infants hospitalized in different neonatal intensive care unit environments. J Pediatr. 2014;164:52–60.e2.
Brown NC, Inder TE, Bear MJ, Hunt RW, Anderson PJ, Doyle LW. Neurobehavior at term and white and gray matter abnormalities in very preterm infants. J Pediatr. 2009;155:32–8.e1.
Pineda RG, Tjoeng TH, Vavasseur C, Kidokoro H, Neil JJ, Inder T. Patterns of altered neurobehavior in preterm infants within the neonatal intensive care unit. J Pediatr. 2013;162:470–6.e1.
Pineda R, Guth R, Herring A, Reynolds L, Oberle S, Smith J. Enhancing sensory experiences for very preterm infants in the NICU: an integrative review. J Perinatol. 2016;37:323–32
Feldman R, Rosenthal Z, Eidelman AI. Maternal-preterm skin-to-skin contact enhances child physiologic organization and cognitive control across the first 10 years of life. Biol Psychiatry. 2014;75:56–64.
Procianoy RS, Mendes EW, Silveira RC. Massage therapy improves neurodevelopment outcome at two years corrected age for very low birth weight infants. Early Hum Dev. 2010;86:7–11.
Juneau AL, Aita M, Heon M. Review and critical analysis of massage studies for term and preterm infants. Neonatal Netw. 2015;34:165–77.
Krueger C. Exposure to maternal voice in preterm infants: a review. Adv Neonatal Care. 2010;10:13–8.
Pineda R, Raney M, Smith J. Supporting and Enhancing NICU Sensory Experiences (SENSE) Intervention Kit. 2018. https://us.bbcollab.com/guest/2283fe483485488fa3fc1f586e0cfb3a.
Pineda R, Roussin J, Heiny E, Smith J. Health care professionals’ perceptions about sensory-based interventions in the NICU. Am J Perinatol. 2018:36;1229–36.
Richter M, Smith J, Pineda R. Health care professional perceptions about a proposed NICU intervention: the importance of community and aligning with everyday occupations. OTJR: Occup Particip Health. 2022:42:238–47.
Lisle JBK, Richter M, Smith J, Satpute P, Pineda R. Maternal perceptions about sensory interventions in the neonatal intensive care unit: an exploratory qualitative study. Front Pediatr. 2022:10;884329.
Pineda R, Wallendorf M, Smith J. A pilot study demonstrating the impact of the supporting and enhancing NICU sensory experiences (SENSE) program on the mother and infant. Early Hum Dev. 2020;144:105000.
Pineda R, Smith J, Roussin J, Wallendorf M, Kellner P, Colditz G. Randomized clinical trial investigating the effect of consistent, developmentally-appropriate, and evidence-based multisensory exposures in the NICU. J Perinatol. 2021:41;2449–62.
Pineda R, Roussin J, Kwon J, Heiny E, Colditz G, Smith J. Applying the RE-AIM framework to evaluate the implementation of the Supporting and Enhancing NICU Sensory Experiences (SENSE) program. BMC Pediatr. 2021;21:137.
Patel D, Thompson J, Hart K, Theese M, Joyce K, Dorton R, et al. Improving caregiver satisfaction and infant feeding outcomes through use of the Supporting and Enhancing NICU Sensory Experiences (SENSE). Am J Occup Ther. 2021;75:7512515325p1.
Deikemper RIB, Merz L. Development of the documentation and appraisal review tool for systematic reviews. World J Meta-Anal. 2013;3:142–50.
National Institute for Health and Care Excellence (NICE). Appendix C: methodology checklist: randomized controlled trials. The Guidelines Manual. 2012. http://publications.nice.org.uk/the-guidelines-manual-appendices-bi-pmg6b/appendix-c-methodology-checklist-randomised-controlled-trials.
Bahman Bijari B, Iranmanesh S, Eshghi F, Baneshi MR. Gentle human touch and Yakson: the effect on preterm’s behavioral reactions. ISRN Nurs. 2012;2012:750363.
Harrison LL, Williams AK, Berbaum ML, Stem JT, Leeper J. Physiologic and behavioral effects of gentle human touch on preterm infants. Res Nurs health. 2000;23:435–46.
Im H, Kim E. Effect of Yakson and gentle human touch versus usual care on urine stress hormones and behaviors in preterm infants: a quasi-experimental study. Int J Nurs Stud. 2009;46:450–8.
Im H, Kim E, Cain KC. Acute effects of Yakson and gentle human touch on the behavioral state of preterm infants. J Child Health Care. 2009;13:212–26.
Chen LL, Su YC, Su CH, Lin HC, Kuo HW. Acupressure and meridian massage: combined effects on increasing body weight in premature infants. J Clin Nurs. 2008;17:1174–81.
Ferber SG, Feldman R, Kohelet D, Kuint J, Dollberg S, Arbel E, et al. Massage therapy facilitates mother–infant interaction in premature infants. Infant Behav Dev. 2005;28:74–81.
Ferber SG, Kuint J, Weller A, Feldman R, Dollberg S, Arbel E, et al. Massage therapy by mothers and trained professionals enhances weight gain in preterm infants. Early Hum Dev. 2002;67:37–45.
Bauer K, Pyper A, Sperling P, Uhrig C, Versmold H. Effects of gestational and postnatal age on body temperature, oxygen consumption, and activity during early skin-to-skin contact between preterm infants of 25-30-week gestation and their mothers. Pediatr Res. 1998;44:247–51.
Bier JA, Ferguson AE, Morales Y, Liebling JA, Archer D, Oh W, et al. Comparison of skin-to-skin contact with standard contact in low-birth-weight infants who are breast-fed. Arch Pediatr Adolesc Med. 1996;150:1265–9.
Bohnhorst B, Heyne T, Peter CS, Poets CF. Skin-to-skin (kangaroo) care, respiratory control, and thermoregulation. J Pediatr. 2001;138:193–7.
de Macedo EC, Cruvinel F, Lukasova K, D’Antino ME. The mood variation in mothers of preterm infants in Kangaroo mother care and conventional incubator care. J Trop Pediatr. 2007;53:344–6.
Feldman R, Eidelman AI, Sirota L, Weller A. Comparison of skin-to-skin (kangaroo) and traditional care: parenting outcomes and preterm infant development. Pediatrics 2002;110:16–26.
Feldman R, Weller A, Sirota L, Eidelman AI. Skin-to-skin contact (Kangaroo care) promotes self-regulation in premature infants: sleep-wake cyclicity, arousal modulation, and sustained exploration. Dev Psychol. 2002;38:194–207.
Fӧhe K, Kropf S, Avenarius S. Skin-to-skin contact improves gas exchange in premature infants. J Perinatol. 2000;20:311–5.
Lee J, Bang K-S. The effects of kangaroo care on maternal self-esteem and premature infants’ physiological stability. Korean J Women Health Nurs. 2011;17:454–62.
Legault M, Goulet C. Comparison of kangaroo and traditional methods of removing preterm infants from incubators. J Obstet Gynecol Neonatal Nurs. 1995;24:501–6.
Maastrup R, Greisen G. Extremely preterm infants tolerate skin-to-skin contact during the first weeks of life. Acta Paediatr. 2010;99:1145–9.
Messmer PR, Rodriguez S, Adams J, Wells-Gentry J, Washburn K, Zabaleta I, et al. Effect of kangaroo care on sleep time for neonates. Pediatr Nurs. 1997;23:408–14.
Miles R, Cowan F, Glover V, Stevenson J, Modi N. A controlled trial of skin-to-skin contact in extremely preterm infants. Early Hum Dev. 2006;82:447–55.
Ramanathan K, Paul VK, Deorari AK, Taneja U, George G. Kangaroo mother care in very low birth weight infants. Indian J Pediatr. 2001;68:1019–23.
Roberts KL, Paynter C, McEwan B. A comparison of kangaroo mother care and conventional cuddling care. Neonatal Netw. 2000;19:31–5.
Rojas MA, Kaplan M, Quevedo M, Sherwonit E, Foster L, Ehrenkranz RA, et al. Somatic growth of preterm infants during skin-to-skin care versus traditional holding: a randomized, controlled trial. J Dev Behav Pediatr. 2003;24:163–8.
Samra NM, Taweel AE, Cadwell K. Effect of intermittent kangaroo mother care on weight gain of low birth weight neonates with delayed weight gain. J Perinat Educ. 2013;22:194–200.
Scher MS, Ludington-Hoe S, Kaffashi F, Johnson MW, Holditch-Davis D, Loparo KA. Neurophysiologic assessment of brain maturation after an 8-week trial of skin-to-skin contact on preterm infants. Clin Neurophysiol. 2009;120:1812–8.
Schneider C, Charpak N, Ruiz-Pelaez JG, Tessier R. Cerebral motor function in very premature-at-birth adolescents: a brain stimulation exploration of kangaroo mother care effects. Acta Paediatr. 2012;101:1045–53.
Smith SL. Physiologic stability of intubated VLBW infants during skin-to-skin care and incubator care. Adv Neonatal Care. 2001;1:28–40.
Smith SL. Heart period variability of intubated very-low-birth-weight infants during incubator care and maternal holding. Am J Crit Care. 2003;12:54–64.
Tallandini MA, Scalembra C. Kangaroo mother care and mother-premature infant dyadic interaction. Infant Ment Health J. 2006;27:251–75.
Tӧrnhage CJ, Serenius F, Uvnas-Moberg K, Lindberg T. Plasma somatostatin and cholecystokinin levels in preterm infants during kangaroo care with and without nasogastric tube-feeding. J Pediatr Endocrinol Metab. 1998;11:645–51.
Coskun D, Gunay U. The effects of kangaroo care applied by Turkish mothers who have premature babies and cannot breastfeed on their stress levels and amount of milk production. J Pediatr Nurs. 2020;50:e26–32.
El-Farrash RA, Shinkar DM, Ragab DA, Salem RM, Saad WE, Farag AS, et al. Longer duration of kangaroo care improves neurobehavioral performance and feeding in preterm infants: a randomized controlled trial. Pediatr Res. 2020;87:683–8.
Shattnawi KK, Al-Ali N. The effect of short duration skin to skin contact on premature infants’ physiological and behavioral outcomes: a quasi-experimental study. J Pediatr Nurs. 2019;46:e24–8.
Forde D, Deming DD, Tan JC, Phillips RM, Fry-Bowers EK, Barger MK, et al. Oxidative stress biomarker decreased in preterm neonates treated with kangaroo mother care. Biol Res Nurs. 2020;22:188–96.
Mehler K, Hucklenbruch-Rother E, Trautmann-Villalba P, Becker I, Roth B, Kribs A. Delivery room skin-to-skin contact for preterm infants—a randomized clinical trial. Acta Paediatr. 2020;109:518–26.
Hucklenbruch-Rother E, Vohlen C, Mehdiani N, Keller T, Roth B, Kribs A, et al. Delivery room skin-to-skin contact in preterm infants affects long-term expression of stress response genes. Psychoneuroendocrinology. 2020;122:104883.
Buil A, Sankey C, Caeymaex L, Apter G, Gratier M, Devouche E. Fostering mother-very preterm infant communication during skin-to-skin contact through a modified positioning. Early Hum Dev. 2020;141:104939.
Buil A, Carchon I, Apter G, Laborne FX, Granier M, Devouche E. Kangaroo supported diagonal flexion positioning: new insights into skin-to-skin contact for communication between mothers and very preterm infants. Arch Pediatr. 2016;23:913–20.
Özdel D, Sarı HY. Effects of the prone position and kangaroo care on gastric residual volume, vital signs and comfort in preterm infants. Jpn J Nurs Sci. 2020;17:e12287.
Diniz KT, Cabral Filho JE, Miranda RM, Lima GMS, Figueredo NPDS, Araújo KFN. Short-time effect of the kangaroo position on electromyographic activity of premature infants: a randomized clinical trial. J Pediatr. 2020;96:741–7.
Sehgal A, Nitzan I, Jayawickreme N, Menahem S. Impact of skin-to-skin parent-infant care on preterm circulatory physiology. J Pediatr. 2020;222:91–7.e2.
Cho ES, Kim SJ, Kwon MS, Cho H, Kim EH, Jun EM, et al. The effects of kangaroo care in the neonatal intensive care unit on the physiological functions of preterm infants, maternal-infant attachment, and maternal stress. J Pediatr Nurs. 2016;31:430–8.
Kurt FY, Kucukoglu S, Ozdemir AA, Ozcan Z. The effect of kangaroo care on maternal attachment in preterm infants. Niger J Clin Pr. 2020;23:26–32.
Dongre S, Desai S, Nanavati R. Kangaroo father care to reduce paternal stress levels: a prospective observational before-after study. J Neonatal Perinat Med. 2020;13:403–11.
Vittner D, McGrath J, Robinson J, Lawhon G, Cusson R, Eisenfeld L, et al. Increase in oxytocin from skin-to-skin contact enhances development of parent-infant relationship. Biol Res Nurs. 2018;20:54–62.
Loewy J. NICU music therapy: song of kin as critical lullaby in research and practice. Ann N Y Acad Sci. 2015;1337:178–85.
Loewy J, Stewart K, Dassler AM, Telsey A, Homel P. The effects of music therapy on vital signs, feeding, and sleep in premature infants. Pediatrics. 2013;131:902–18.
Cassidy JW. The effect of decibel level of music stimuli and gender on head circumference and physiological responses of premature infants in the NICU. J Music Ther. 2009;46:180–90.
Farhat A, Amiri R, Karbandi S, Esmaily H, Mohammadzadeh A. The effect of listening to lullaby music on physiologic response and weight gain of premature infants. J Neonatal Perinat Med. 2010;3:103–7.
Keidar HR, Mandel D, Mimouni FB, Lubetzky R. Bach music in preterm infants: no ‘Mozart effect’ on resting energy expenditure. J Perinatol. 2014;34:153–5.
Krueger C, Parker L, Chiu SH, Theriaque D. Maternal voice and short-term outcomes in preterm infants. Dev Psychobiol. 2010;52:205–12.
Picciolini O, Porro M, Meazza A, Gianni ML, Rivoli C, Lucco G. Early exposure to maternal voice: effects on preterm infants development. Early Hum Dev. 2014;90:287–92.
Webb AR, Heller HT, Benson CB, Lahav A. Mother’s voice and heartbeat sounds elicit auditory plasticity in the human brain before full gestation. Proc Natl Acad Sci USA. 2015;112:3152–7.
Zimmerman E, Keunen K, Norton M, Lahav A. Weight gain velocity in very low-birth-weight infants: effects of exposure to biological maternal sounds. Am J Perinatol. 2013;30:863–70.
Nöcker-Ribaupierre M, Linderkamp O, Riegel K. The effects of mothers’ voice on the long term development of premature infants: a prospective randomized study. Music Med. 2015;7:20–5.
Lejeune F, Lordier L, Pittet MP, Schoenhals L, Grandjean D, Huppi PS, et al. Effects of an early postnatal music intervention on cognitive and emotional development in preterm children at 12 and 24 months: preliminary findings. Front Psychol. 2019;10:494.
Lordier L, Meskaldji DE, Grouiller F, Pittet MP, Vollenweider A, Vasung L, et al. Music in premature infants enhances high-level cognitive brain networks. Proc Natl Acad Sci USA. 2019;116:12103–8.
Sa de Almeida J, Lordier L, Zollinger B, Kunz N, Bastiani M, Gui L, et al. Music enhances structural maturation of emotional processing neural pathways in very preterm infants. Neuroimage. 2020;207:116391.
Lordier L, Loukas S, Grouiller F, Vollenweider A, Vasung L, Meskaldij DE, et al. Music processing in preterm and full-term newborns: a psychophysiological interaction (PPI) approach in neonatal fMRI. Neuroimage. 2019;185:857–64.
Lejeune F, Brand LA, Palama A, Parra J, Marcus L, Barisnikov K, et al. Preterm infant showed better object handling skills in a neonatal intensive care unit during silence than with a recorded female voice. Acta Paediatr. 2019;108:460–7.
Ranger A, Helmert E, Bott TS, Ostermann T, Als H, Bassler D, et al. Physiological and emotional effects of pentatonic live music played for preterm neonates and their mothers in the Newborn Intensive Care Unit: a randomized controlled trial. Complement Ther Med. 2018;41:240–6.
Detmer MR, Evans K, Shina E, Walker K, DeLoach D, Malowitz JR. Multimodal neurologic enhancement improves preterm infants’ developmental outcomes: a longitudinal pilot study. Neonatal Netw. 2020;39:16–23.
Jabraeili M, Sabet T, MustafaGharebaghi M, Asghari Jafarabadi M, Arshadi M. The effect of recorded Mum’s Lullaby and Brahm’s Lullaby on oxygen saturation in preterm infants: a randomized double-blind clinical trial. J Caring Sci. 2016;5:85–93.
Etzel R, Balk S, Bearer C, Miller M, Shea K, Simon P. Noise: a hazard for the fetus and newborn. Pediatrics. 1997;1997:724–7.
Shafiei E, Ameri ZD, Sheikhbardsiri H, Yaseri M, Baniasadi H. The effect of mother’s lullaby on preterm infants’ physiological parameters. J Pediatr Res. 2020;7:46–51.
Guyer C, Huber R, Fontijn J, Bucher HU, Nicolai H, Werner H, et al. Very preterm infants show earlier emergence of 24-h sleep-wake rhythms compared to term infants. Early Hum Dev. 2015;91:37–42.
Morag I, Ohlsson A. Cycled light in the intensive care unit for preterm and low birth weight infants. Cochrane Database Syst Rev. 2013:8;CD006982.
Vásquez-Ruiz S, Maya-Barrios JA, Torres-Narvaez P, Vega-Martinez BR, Rojas-Granados A, Escobar C, et al. A light/dark cycle in the NICU accelerates body weight gain and shortens time to discharge in preterm infants. Early Hum Dev. 2014;90:535–40.
Brandon DH, Silva SG, Park J, Malcolm W, Kamhawy H, Holditch-Davis D. Timing for the introduction of cycled light for extremely preterm infants: a randomized controlled trial. Res Nurs Health. 2017;40:294–310.
Lebel V, Aita M, Johnston C, Heon M, Dupuis F. Effects of cycled lighting versus continuous near darkness on physiological stability and motor activity level in preterm infants. Adv Neonatal Care. 2017;17:282–91.
Morag I, Ohlsson A. Cycled light in the intensive care unit for preterm and low birth weight infants. Cochrane Database Syst Rev. 2016;2016:Cd006982.
Kucuk Alemdar D, Inal S. The effect of individualized developmental care practices in preterm infants. Complement Med Res. 2020;27:97–104.
Fontana C, De Carli A, Ricci D, Dessimone F, Passera S, Pesenti N, et al. Effects of early intervention on visual function in preterm infants: a randomized controlled trial. Front Pediatr. 2020;8:291.
Kaneshi Y, Ohta H, Morioka K, Hayasaka I, Uzuki Y, Akimoto T, et al. Influence of light exposure at nighttime on sleep development and body growth of preterm infants. Sci Rep. 2016;6:21680.
Schulzke S, Trachsel D, Patole S. Physical activity programs for promoting bone mineralization and growth in preterm infants. Cochrane Database Syst Rev. 2014:4;CD005387.
Vignochi CM, Silveira RC, Miura E, Canani LH, Procianoy RS. Physical therapy reduces bone resorption and increases bone formation in preterm infants. Am J Perinatol. 2012;29:573–8.
Sezer Efe Y, Erdem E, Gunes T. The effect of daily exercise program on bone mineral density and cortisol level in preterm infants with a very low birth weight: a randomized controlled trial. J Pediatr Nurs. 2020:51;e6–12.
Fjortoft T, Ustad T, Follestad T, Kaaresen PI, Oberg GK. Does a parent-administrated early motor intervention influence general movements and movement character at 3 months of age in infants born preterm? Early Hum Dev. 2017;112:20–4.
Ustad T, Evensen KA, Campbell SK, Girolami GL, Helbostad J, Jorgensen L, et al. Early parent-administered physical therapy for preterm infants: a randomized controlled trial. Pediatrics. 2016:138;1–8.
Oberg GK, Girolami GL, Campbell SK, Ustad T, Heuch I, Jacobsen BK, et al. Effects of a parent-administered exercise program in the neonatal intensive care unit: dose does matter—a randomized controlled trial. Phys Ther. 2020;100:860–9.
Aldana Acosta A, Tessier R, Charpak N, Tarabulsy G. Randomised controlled trial on the impact of kinesthetic stimulation on early somatic growth of preterm infants in Kangaroo position. Acta Paediatr. 2018;108:1230–6.
Baby S, Sangeethajanani SA, Kanchana S. Effect of tactile-kinesthetic stimulation on neuromuscular maturity among pre-term infants at selected hospital Chennai. Nurs J India. 2015:106;258–60.
Baniasadi H, Hosseini SS, Abdollahyar A, Sheikhbardsiri H. Effect of massage on behavioural responses of preterm infants in an educational hospital in Iran. J Reprod Infant Psychol. 2019;37:302–10.
Elmoneim MA, Mohamed HA, Awad A, El-Hawary A, Salem N, El Helaly R, et al. Effect of tactile/kinesthetic massage therapy on growth and body composition of preterm infants. Eur J Pediatr. 2021;180:207–15.
Álvarez MJ, Rodríguez-González D, Rosón M, Lapeña S, Gómez-Salgado J, Fernández-García D. Effects of massage therapy and kinesitherapy to develop hospitalized preterm infant’s anthropometry: a quasi-experimental study. J Pediatr Nurs. 2019;46:e86–91.
Taheri PA, Goudarzi Z, Shariat M, Nariman S, Matin EN. The effect of a short course of moderate pressure sunflower oil massage on the weight gain velocity and length of NICU stay in preterm infants. Infant Behav Dev. 2018;50:22–7.
Choi H, Kim SJ, Oh J, Lee MN, Kim S, Kang KA. The effects of massage therapy on physical growth and gastrointestinal function in premature infants: a pilot study. J Child Health Care. 2015:20;394–404.
Kardas Ozdemir F, Guducu, Tufekci F. The effect of individualised developmental care practices on the growth and hospitalisation duration of premature infants: the effect of mother’s scent and flexion position. J Clin Nurs. 2014;23:3036–44.
Lee J, Kim HS, Jung YH, Choi KY, Shin SH, Kim EK, et al. Oropharyngeal colostrum administration in extremely premature infants: an RCT. Pediatrics. 2015;135:e357–66.
Yildiz A, Arikan D, Gozum S, Tastekin A, Budancamanak I. The effect of the odor of breast milk on the time needed for transition from gavage to total oral feeding in preterm infants. J Nurs Scholarsh. 2011;43:265–73.
Russell K, Weaver B, Vogel R. Neuroprotective core measure 2: partnering with families—effects of a weighted maternally-scented parental simulation device on premature infants in neonatal intensive care. Newborn Infant Nurs Rev. 2015;15:97–103.
Konar MC, Islam K, Roy A, Ghosh T. Effect of virgin coconut oil application on the skin of preterm newborns: a randomized controlled trial. J Trop Pediatr. 2020;66:129–35.
Aly H, Moustafa MF, Hassanein SM, Massaro AN, Amer HA, Patel K. Physical activity combined with massage improves bone mineralization in premature infants: a randomized trial. J Perinatol. 2004;24:305–9.
Ang JY, Lua JL, Mathur A, Thomas R, Asmar BI, Savasan S, et al. A randomized placebo-controlled trial of massage therapy on the immune system of preterm infants. Pediatrics. 2012;130:e1549–58.
Arnon S, Diamant C, Bauer S, Regev R, Sirota G, Litmanovitz I. Maternal singing during kangaroo care led to autonomic stability in preterm infants and reduced maternal anxiety. Acta Paediatr. 2014;103:1039–44.
Cameron EC, Maehle V, Reid J. The effects of an early physical therapy intervention for very preterm, very low birth weight infants: a randomized controlled clinical trial. Pediatr Phys Ther. 2005;17:107–19.
Diego MA, Field T, Hernandez-Reif M. Vagal activity, gastric motility, and weight gain in massaged preterm neonates. J Pediatr. 2005;147:50–5.
Diego MA, Field T, Hernandez-Reif M. Temperature increases in preterm infants during massage therapy. Infant Behav Dev. 2008;31:149–52.
Diego MA, Field T, Hernandez-Reif M. Preterm infant weight gain is increased by massage therapy and exercise via different underlying mechanisms. Early Hum Dev. 2014;90:137–40.
Diego MA, Field T, Hernandez-Reif M, Deeds O, Ascencio A, Begert G. Preterm infant massage elicits consistent increases in vagal activity and gastric motility that are associated with greater weight gain. Acta Paediatr. 2007;96:1588–91.
Dieter JN, Field T, Hernandez-Reif M, Emory EK, Redzepi M. Stable preterm infants gain more weight and sleep less after five days of massage therapy. J Pediatr Psychol. 2003;28:403–11.
Field T, Diego M, Hernandez-Reif M, Dieter JN, Kumar AM, Schanberg S, et al. Insulin and insulin-like growth factor-1 increased in preterm neonates following massage therapy. J Dev Behav Pediatr. 2008;29:463–6.
Field T, Diego MA, Hernandez-Reif M, Deeds O, Figuereido B. Moderate versus light pressure massage therapy leads to greater weight gain in preterm infants. Infant Behav Dev. 2006;29:574–8.
Gonzalez AP, Vasquez-Mendoza G, Garcia-Vela A, Guzman-Ramirez A, Salazar-Torres M, Romero-Gutierrez G. Weight gain in preterm infants following parent-administered Vimala massage: a randomized controlled trial. Am J Perinatol. 2009;26:247–52.
Haley S, Beachy J, Ivaska KK, Slater H, Smith S, Moyer-Mileur LJ. Tactile/kinesthetic stimulation (TKS) increases tibial speed of sound and urinary osteocalcin (U-MidOC and unOC) in premature infants (29–32 weeks PMA). Bone. 2012;51:661–6.
Hane AA, Myers MM, Hofer MA, Ludwig RJ, Halperin MS, Austin J, et al. Family nurture intervention improves the quality of maternal caregiving in the neonatal intensive care unit: evidence from a randomized controlled trial. J Dev Behav Pediatr. 2015;36:188–96.
Hernandez-Reif M, Diego M, Field T. Preterm infants show reduced stress behaviors and activity after 5 days of massage therapy. Infant Behav Dev. 2007;30:557–61.
Holditch-Davis D, White-Traut RC, Levy JA, O’Shea TM, Geraldo V, David RJ. Maternally administered interventions for preterm infants in the NICU: effects on maternal psychological distress and mother-infant relationship. Infant Behav Dev. 2014;37:695–710.
Kanagasabai PS, Mohan D, Lewis LE, Kamath A, Rao BK. Effect of multisensory stimulation on neuromotor development in preterm infants. Indian J Pediatr. 2013;80:460–4.
Massaro AN, Hammad TA, Jazzo B, Aly H. Massage with kinesthetic stimulation improves weight gain in preterm infants. J Perinatol. 2009;29:352–7.
Mathai S, Fernandez A, Mondkar J, Kanbur W. Effects of tactile-kinesthetic stimulation in preterms: a controlled trial. Indian Pediatr. 2001;38:1091–8.
Matricardi S, Agostino R, Fedeli C, Montirosso R. Mothers are not fathers: differences between parents in the reduction of stress levels after a parental intervention in a NICU. Acta Paediatr. 2013;102:8–14.
Mendes EW, Procianoy RS. Massage therapy reduces hospital stay and occurrence of late-onset sepsis in very preterm neonates. J Perinatol. 2008;28:815–20.
Moyer-Mileur LJ, Haley S, Slater H, Beachy J, Smith SL. Massage improves growth quality by decreasing body fat deposition in male preterm infants. J Pediatr. 2013;162:490–5.
Schlez A, Litmanovitz I, Bauer S, Dolfin T, Regev R, Arnon S. Combining kangaroo care and live harp music therapy in the neonatal intensive care unit setting. Isr Med Assoc J. 2011;13:354–8.
Smith SL, Lux R, Haley S, Slater H, Beachy J, Beechy J, et al. The effect of massage on heart rate variability in preterm infants. J Perinatol. 2013;33:59–64.
Standley JM. The effect of music and multimodal stimulation on responses of premature infants in neonatal intensive care. Pediatr Nurs. 1998;24:532–8.
Teckenberg-Jansson P, Huotilainen M, Polkki T, Lipsanen J, Jarvenpaa A. Rapid effects of neonatal music therapy combined with kangaroo care on prematurely-born infants. Nord J Music Ther 2011;20:22–42.
Valizadeh S, Hosseini MB, Asghari Jafarabadi M, Ajoodanian N. The effects of massage with coconut and sunflower oils on oxygen saturation of premature infants with respiratory distress syndrome treated with nasal continuous positive airway pressure. J Caring Sci. 2012;1:191–9.
Welch MG, Hofer MA, Stark RI, Andrews HF, Austin J, Glickstein SB, et al. Randomized controlled trial of Family Nurture Intervention in the NICU: assessments of length of stay, feasibility and safety. BMC Pediatr. 2013;13:148.
White-Traut R, Norr KF, Fabiyi C, Rankin KM, Li Z, Liu L. Mother-infant interaction improves with a developmental intervention for mother-preterm infant dyads. Infant Behav Dev. 2013;36:694–706.
White-Traut R, Rankin KM, Pham T, Li Z, Liu L. Preterm infants’ orally directed behaviors and behavioral state responses to the integrated H-HOPE intervention. Infant Behav Dev. 2014;37:583–96.
White-Traut RC, Nelson MN, Silvestri JM, Cunningham N, Patel M. Responses of preterm infants to unimodal and multimodal sensory intervention. Pediatr Nurs. 1997;23:169–75.
White-Traut RC, Nelson MN, Silvestri JM, Patel M, Berbaum M, Gu GG, et al. Developmental patterns of physiological response to a multisensory intervention in extremely premature and high-risk infants. J Obstet Gynecol Neonatal Nurs. 2004;33:266–75.
White-Traut RC, Nelson MN, Silvestri JM, Patel M, Vasan U, Han BK, et al. Developmental intervention for preterm infants diagnosed with periventricular leukomalacia. Res Nurs Health. 1999;22:131–43.
White-Traut RC, Nelson MN, Silvestri JM, Vasan U, Littau S, Meleedy-Rey P, et al. Effect of auditory, tactile, visual, and vestibular intervention on length of stay, alertness, and feeding progression in preterm infants. Dev Med Child Neurol. 2002;44:91–7.
White-Traut RC, Rankin KM, Yoder JC, Liu L, Vasa R, Geraldo V, et al. Influence of H-HOPE intervention for premature infants on growth, feeding progression and length of stay during initial hospitalization. J Perinatol. 2015;35:636–41.
Vahdati M, Mohammadizadeh M, Talakoub S. Effect of kangaroo care combined with music on the mother-premature neonate attachment: a randomized controlled trial. Iran J Nurs Midwifery Res. 2017;22:403–7.
Ettenberger M, Rojas Cardenas C, Parker M, Odell-Miller H. Family-centred music therapy with preterm infants and their parents in the Neonatal Intensive Care Unit (NICU) in Colombia—a mixed-methods study. Nord J Music Ther. 2017;26:207–34.
Efendi D, Caswini N, Rustina Y, Iskandar R. Combination of Mother Therapeutic Touch (MTT) and Maternal Voice Stimulus (MVS) therapies stabilize sleep and physiological function in preterm infants receiving minor invasive procedures. J Neonatal Nurs. 2018;24:318–24.
Jaywant S, Dandavate P, Kale J. Premature infant oral motor intervention (PIOMI) with and without massage therapy on social emotional development in preterm infants. Indian J Occup Ther. 2020;52:95–100.
Singal AG, Higgins PD, Waljee AK. A primer on effectiveness and efficacy trials. Clin Transl Gastroenterol. 2014;5:e45.
Wiltsey Stirman S, Baumann AA, Miller CJ. The FRAME: an expanded framework for reporting adaptations and modifications to evidence-based interventions. Implement Sci. 2019;14:58.
Acknowledgements
The project was supported by the Eunice Kennedy Shriver National Institute of Child Health and Human Development (U54 HD087011) to the Intellectual and Developmental Disabilities Research Center at Washington University and the Washington University Institute of Clinical and Translational Sciences Clinical and Translational Funding Program (National Institutes of Health/National Center for Advancing Translational Sciences UL1 TR000448). We would like to thank Jessica Roussin, Jenny Kwon, Marinthea Richter, Bethany Gruskin, Delaney Smith, and Prutha Satpute as well as all the neonatal practitioners who work toward improving care for high-risk infants in the NICU.
Funding
Open access funding provided by SCELC, Statewide California Electronic Library Consortium.
Author information
Authors and Affiliations
Contributions
RP conceived of the original idea to do an integrative review to inform a clinical practice guideline on sensory-based interventions in the NICU. She was involved with data synthesis, and wrote the first draft of the manuscript. She oversaw all parts of the project, and approved the final version of the manuscript submitted. RG and AG conducted the literature review and identified articles appropriate for the integrative review. They were involved in identifying the articles, assessing each for quality and wrote the first draft of the evidence table. They critically reviewed the manuscript’s content and approved the final version of the manuscript submitted. PK assisted with the synthesis of articles, assisted with drafts and revisions of the manuscript, and approved the final manuscript as submitted. JS was involved in idea conception, study design, data synthesis, and ensured accuracy of the studies reported. She oversaw all parts of the project. She provided intellectual content to the manuscript and approved the final version that was submitted.
Corresponding author
Ethics declarations
Competing interests
JS and RP are authors of the SENSE program. The program is available “at cost” to other NICUs with no direct financial benefit to JS and RP.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Pineda, R., Kellner, P., Guth, R. et al. NICU sensory experiences associated with positive outcomes: an integrative review of evidence from 2015–2020. J Perinatol 43, 837–848 (2023). https://doi.org/10.1038/s41372-023-01655-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41372-023-01655-y