Abstract
Background
Donor breast milk (DBM) feeding has been associated with less growth than formula in preterm infants. Zinc content in DBM is insufficient to support growth in preterm infants.
Objective
To compare growth from birth to discharge, macro- and micronutrient intake and the frequency of poor growth before (Epoch-1) and after (Epoch-2) implementing a DBM program.
Methods
Retrospective cohort study of 1069 infants born at < 33 weeks’ gestational age or birthweight < 1500 g and fed using our adjustable feeding protocol with accurate serial length measurements. Growth was assessed by changes in Z-scores of weight, length and fronto-occipital circumference from birth to discharge.
Results
Growth did not decrease significantly in Epoch-2. However, energy and protein intake increased by 5% and frequency of zinc and vitamin D supplementation increased by >30%.
Conclusions
DBM implementation did not significantly decrease growth from birth to discharge using our adjustable feeding protocol.
Similar content being viewed by others
Introduction
Breast milk (BM) is recommended for feeding preterm infants [1, 2]. Although mother’s own milk (MOM) has numerous possible short- and long-term benefits, including reducing rates of late onset sepsis (LOS), necrotizing enterocolitis (NEC) and improved neurodevelopmental outcomes [1,2,3], this evidence is limited by lack of randomized controlled trials [4,5,6,7]. Systematic reviews of all available observational studies have consistently shown only benefit of MOM in reducing NEC [5]. In contrast, several studies using propensity score analysis or dose-dependent analysis of MOM intake have shown multiple short-term and neurodevelopmental benefits of MOM in very low birth weight (VLBW) or very preterm infants [8]. The protective effect of human milk on NEC is not completely understood but has been hypothesized to be multifactorial and related to bioactive components in MOM [9].
Mothers of preterm neonates often have difficulty establishing a full supply of BM for their baby, requiring alternative nutritional sources, such as preterm formula and pasteurized donor breast milk (DBM). The American Academy of Pediatrics recommends DBM plus a multinutrient fortifier as the first alternative to MOM for VLBW infants [1]. DBM was available for VLBW infants in 87% of United States hospitals with level III or IV neonatal intensive care units (NICUs) in 2020 [10].
DBM exhibits some of the same health benefits of MOM for preterm infants, including decreased risk of NEC compared with preterm formula [11, 12]. However, there are some concerns associated with DBM feeding, including deactivation of many of the biologically active components and elimination of potentially beneficial bacteria by pasteurization, and suboptimal infant growth rates when compared to formula [11, 13,14,15]. Implementation of a DBM program may result in decreased MOM feeding secondary to less pumping due to false interpretation that DBM is a replacement for MOM rather than a supplement until MOM production increases [15]. Macronutrient content of human milk is dependent on many factors including gestational age (GA) and stage of lactation; term milk contains less energy and protein when compared to preterm milk [16, 17]. Most commonly, DBM is from mothers of term infants at later stages of lactation [16]. Even though human milk banks may utilize target-pooling of milk to reduce nutritional variability and increase nutritional content to achieve a minimum caloric density of 20 kcal/oz, protein concentration is often inadequate [18] to support growth for preterm infants. According to the European Society for Pediatric Gastroenterology Hepatology and Nutrition, the recommended protein intake for extremely low birth weight (BW) preterm infants ranges from about 4.0 to 4.5 g/kg/day [19]. Protein content in preterm MOM ranges from 1.4 to 2.2 g/dL in the first four weeks of lactation [17] while DBM protein content is generally about 0.9 g/dL [16].
DBM also differs from MOM in terms of zinc (Zn) content. The concentration of Zn in DBM is lower than in milk of women delivering prematurely and is insufficient to support growth in preterm infants [20, 21]. Additionally, preterm infants are at increased risk of Zn deficiency because of lower stores, secondary to shortened gestational period, and increased requirements [22, 23]. According to recently published systematic reviews, Zn supplementation in preterm infants may improve weight gain and linear growth, as well as decrease mortality [22, 24].
Vitamin D is another micronutrient that differs in term and preterm milk. In general, vitamin D content in human milk is low, even for lactating mothers with adequate vitamin status [25], and as such the current recommendation is for all breastfed infants to receive vitamin D supplementation. Term human milk contains about 1.5–3 IU/dL of vitamin D while preterm human milk contains about 5–8 IU/dL and decreases as lactation progresses [26, 27]. Vitamin D levels in DBM are further reduced by the pasteurization process, which may decrease levels by 10–20% [25]. Growth failure may be one of the early signs of rickets, caused by vitamin D deficiency [28]. Several studies have examined the effect of vitamin D supplementation on infant growth and suggest that early and higher doses of supplementation are associated with better length and head circumference growth [29,30,31].
The objective of this study was to compare growth from birth to discharge before and after implementation of the DBM program as well as to determine the frequency of poor growth associated with Zn and vitamin D deficiency. The hypothesis was that there would be decreased growth and increased frequency of insufficient growth after implementation of the DBM program. We also hypothesized that decreased growth with DBM could result from lower content of micronutrients compared to formula.
Materials/Subjects and methods
Study design and setting
This was a retrospective cohort study of infants born at the 96-bed, Level III NICU at Parkland Hospital, Dallas, TX between January 2018 and December 2021. A DBM program was implemented in January 2020 as part of a quality improvement (QI) project aimed at reducing the incidence of NEC. Inborn infants with GA < 33 weeks or BW < 1500 g were eligible for DBM until 36 weeks postmenstrual age (PMA) upon signed consent by the mother. DBM was obtained from the Mother’s Milk Bank of North Texas, which provided DBM with 20 cal/oz and 1 g/dL protein as measured using Fourier-transformed infrared spectroscopy. The period between January 2018-December 2019 is termed Epoch-1, while the period between January 2020-December 2021 is termed Epoch-2.
Since DBM implementation may result in reduction of percentage of feeding MOM, a QI project was implemented a year before DBM implementation to increase MOM production. This project included educational meetings for personnel involved in pregnancy, delivery room, postpartum and NICU care, purchase of breast pumps, refrigerators, and special attention of lactation consultants to educate mothers to start pumping as early as possible after delivery. Unfortunately, DBM implementation was followed by the SARS-CoV-2 pandemic, which resulted in limitation of family visitations and of face-to-face contact of lactation consultants with mothers for several months.
The feeding guidelines and feeding adjustment protocol based on growth patterns and serial accurate and precise measurements of length (using a length board or a caliper rather than tape measure) and serum concentrations of selected nutrients are presented in Supplementary Tables 1 and 2, which include an update of previously published material [32, 33]. Human milk was fortified as previously described [32, 33]. Similac Human Milk Fortifier Hydrolyzed Protein Concentrated liquid has 0.31 mg Zn per 5 ml packet; this yielded 1.32 mg Zn/dL of fortified breast milk when mixed to 334.7 joules/dL (24 kcal/oz). Serum Zn concentration was measured in non-hemolyzed samples in infants 1) with excessive decrease in length Z-scores or weight and length Z-scores from birth as defined previously [32] despite our adjustable feeding approach [33] or 2) who had risk factors for Zn deficiency (e.g., short bowel syndrome). Serum concentration of 25-hydroxy vitamin D was measured in infants with poor growth or poor linear growth, high serum level of alkaline phosphatase or osteopenia of prematurity diagnosed on radiographs. Measurements of other micronutrients were done only for specific indications in individual patients (Supplementary Table 1).
Inclusion and exclusion criteria
Inborn infants with GA < 33 weeks or BW < 1500 g were included in the study. Infants who received comfort care only were excluded from the study. For growth data, infants who had major congenital anomalies which may alter growth (e.g., trisomy 18 or 21) and those who died prior to discharge were also excluded.
Variables
Zn concentration in pooled DBM is about half that in preterm milk in the US [21], whereas only minimal differences in concentrations of other trace elements have been reported between term and preterm human milk [34]. In order to verify that Zn levels in DBM were as low as expected from the literature [12, 13, 21], Zn concentration was measured in a convenience sample (n = 11 separate aliquots) of pooled DBM collected soon after implementing the DBM program. Since Zn and copper (Cu) compete for gastrointestinal absorption [35] we also measured Cu concentration in DBM. Milk samples were analyzed using Agilent 4210 microwave plasma-atomic emission spectroscopy (MP-AES 4120, Agilent, Santa Clara, CA).
Demographic information on mothers and infants was obtained from three long-standing prospective databases: the Maternal Fetal Medicine Database, the Neonatal Resuscitation Database, the previously validated NICU Database [36, 37], and from direct extraction from the electronic health record (EHR). Data included maternal data (race/ethnicity, body mass index (BMI), chronic hypertension and other pregnancy complications) and neonatal data at birth (GA, BW, birth length, birth fronto-occipital circumference (FOC), Apgar scores, temperature at admission, among others), morbidity (including sepsis episodes, NEC stage II or greater [classified using modified Bell’s criteria [38], and mechanical ventilation) and mortality during initial hospitalization.
Feeding type (MOM, DBM or preterm formula) and days of each feed type were recorded until 36 weeks’ PMA. A cut-off of 36 weeks’ PMA was chosen given that infants are transitioned off DBM at this age. Prolonged MOM feeding was defined as still feeding MOM at the mean age at which NEC developed for each GA group at Parkland Hospital for high-risk infants born from 2009 until 2021 (32 weeks’ PMA if GA < 29 weeks; 34 weeks PMA if GA 29–32 week; 14 days postnatal age if GA ≥ 33 weeks). Delayed formula introduction was defined as formula introduced only after the mean age of NEC for each GA category. Additional information on energy and protein intake, days of total parenteral nutrition, and days to reach full feeds (120–160 ml/kg/d) were collected electronically from discrete data fields in the EHR. MOM protein and energy content was assumed based on published data on preterm (1.4 g/100 ml and 66 cal/100 ml) and term (1.0 g/100 ml and 18.7 cal/oz or 62.3 cal/100 ml) human milk [17, 26, 39].
Growth was assessed by the change (Delta) in Z-scores of weight, length and FOC from birth to discharge [32, 33]. The primary outcome was the change in Z-score of accurate length from birth to discharge. Analyses included comparisons of unadjusted data and of data adjusted for sex and GA.
Patient growth charts were reviewed from the EHR to classify growth patterns. Growth patterns were analyzed in the first 4 weeks of life and subsequently from then until discharge. The weight loss pattern within the first 4 weeks of life was compared to the post-natal growth patterns published by Rochow [40] to assess if the patient had lost an appropriate amount of weight or had excessive weight gain or loss during this period. The subsequent growth pattern from 4 weeks of life to discharge was classified as physiologic growth (where both weight and length growth are sufficient), insufficient growth (where both length and weight are poor), insufficient linear growth, insufficient weight gain or excess weight gain [32].
Zn deficiency was defined as serum Zn concentration <0.74 mcg/mL [41]. Zn deficiency was treated either enterally with Zn chloride at 3.5 mg/kg/day of Zn and Cu at 350 mcg/kg/day in 2 doses (Zn separate time from Cu) or intravenously with Zn chloride at 650 mcg/kg/day of Zn. A repeat serum Zn concentration was measured within a month. Since Cu concentration in DBM was similar to that in preterm human milk and since we provided a Zn:Cu ratio of 10:1 (lower than the recommended maximum molar ratio of 20:1) [35], serum Cu concentration was only measured if necessary. Vitamin D deficiency was defined as a 25-hydroxy vitamin D concentration of < 20 ng/mL, while a level of 20–29 ng/mL was considered as insufficient. Vitamin D deficiency or insufficiency was treated with enteral cholecalciferol to achieve a total of 2000 units/day in 4 doses. A repeat serum concentration was measured within a month.
Statistical analysis
Statistical analyses were conducted using SPSS version 23 (IBM, Inc., Armonk, NY) and included Student t-test, paired Student t-test, ANOVA, logistic regression analysis and Cox regression. We estimated that 676 infants (338 in each group) would be sufficient to detect an effect size of 0.25 (i.e., a difference in Delta Z-score birth to discharge) from −1 to −0.75 with a standard deviation (SD) of 1 [41] using Student t-test with a power of 0.9 and an alpha error of < 0.05. We planned to analyze data on 1000 infants to adjust for exclusions at birth and death prior to discharge.
IRB approval and registration
The Institutional Review Board of University of Texas Southwestern Medical Center and Parkland Health and Hospital Systems approved the study and waived the need for individual consent.
Results
The cohort included a total of 914 mothers, 465 in Epoch-1 and 449 in Epoch-2 and their 1069 infants, 541 infants in Epoch-1 and 528 in Epoch-2 (Supplementary Fig. 1). Maternal and neonatal characteristics are summarized in Table 1. Maternal characteristics were similar in the two epochs. Mean admission temperature decreased in Epoch-2 because of more frequent hypothermia and less frequent hyperthermia (P < 0.001). Accurate length Z-score within the first week of life was lower in neonates born during Epoch-2 (−0.27 ± 0.98 in Epoch-1 vs −0.41 ± 0.92 in Epoch-2, P = 0.04). Neonatal morbidities were similar between both epochs except for mechanical ventilation which was less frequent during Epoch-2 (P < 0.001) (Supplementary Table 3).
Zinc and copper content in DBM samples
The Zn concentration measured in the 11 DBM samples of DBM from the Mother’s Milk Bank of North Texas was 2.14 ± 0.73 mcg/mL (mean ± SD, n = 11) (range = 1.09–3.02 mcg/mL). That value was similar to published data on DBM collected at 2–4 months postnatal in mothers delivering at term, but lower than in preterm human milk [20, 42, 43]. The 11 DBM samples of DBM from the Mother’s Milk Bank of North Texas had a Cu concentration of 0.40 ± 0.05 mcg/mL (range = 0.34–0.50 mcg/mL). That value was similar to term and preterm human milk [44, 45]. These results were presented in seminars to neonatologists, advanced practitioners and dietitians within the Division of Neonatal-Perinatal Medicine, to increase their awareness of risk of Zn deficiency with DBM implementation.
Feeding milk type, intake and duration
The feeding milk types until 36 weeks’ PMA among infants who received enteral feeding are summarized in Table 2. In general, infants were less likely to receive any formula during Epoch-2 (47% vs 93%, P < 0.001) and more likely to receive any DBM (81% vs 4%, P < 0.001). There was no difference between epochs in the percentage of infants receiving exclusive MOM. Additionally, we did not observe any significant differences between epochs in the length of time infants received MOM (Supplementary Fig. 2).
Table 2 summarizes the nutritional intake during both epochs. Protein and energy intake increased by 5% from Epoch-1 to Epoch-2; this increase was observed both during the first 4 weeks and beyond. The time to reach full enteral feeding did not change significantly (P = 0.80).
Growth outcomes
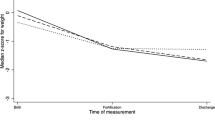
In the entire cohort, there were no significant differences between epochs in postnatal weight loss or unadjusted Delta Z-scores for weight, length and FOC growth from birth to discharge (Table 3A). There were also no significant differences in adjusted delta z-scores for weight, length, or FOC.
Accurate length measurements at birth and discharge were available in 782/955 (82%) infants (Table 3B). Infants with accurate length measurements had smaller GA and BW than those who did not (30.0 ± 2.6 vs 31.0 ± 2.6 weeks, P < 0.001 and 1369 ± 306 vs 1706 ± 530, P < 0.001, respectively). Birth length measurements obtained with the measuring tape were significantly greater than corresponding length measurements obtained by dietitians utilizing the length board or caliper (Z-scores −0.09 ± 1.04 vs −0.33 ± 0.95, n = 781, P < 0.001). The adjusted Delta Z-score for length from birth to discharge did not change significantly from Epoch-1 to Epoch-2, whether measured using length board or caliper (P = 0.08) or tape measure (P = 0.48).
Growth was also assessed by analyzing the growth patterns from the patient’s growth charts (Table 4). There were no significant differences in the initial weight loss patterns between epochs. Several infants were discharged home within the first four weeks of life, explaining lower numbers of infants after that. The only significant difference between growth patterns after the first four weeks of life in the entire cohort was a lower percentage of infants with insufficient growth during Epoch-2 vs Epoch-1 (15% vs 23%, respectively, P = 0.02).
Zinc
Serum Zn concentrations were measured at similar frequency in both epochs but at younger PMA during Epoch-2 when compared to Epoch-1 (34.3 weeks vs 36.1 weeks, P = 0.01) (Table 5). Infants who had Zn concentration measured were more likely to have growth failure or insufficient weight gain than those who did not have it measured and less likely to have a physiologic growth pattern (Supplementary Table 4).
The frequency of diagnosis of Zn deficiency increased from 10% in Epoch-1 to 14% in Epoch-2 (P = 0.04). The frequency of diagnosis of combined Zn and vitamin D deficiency increased from 3% in Epoch-1 to 7% in Epoch-2 (P < 0.05). Factors associated with Zn deficiency included lower GA (P < 0.001) and later introduction of formula (P < 0.001) (Supplementary Table 5). Among infants with Zn deficiency there were no significant differences in the average energy intake, protein, or Zn intake at the time of diagnosis of Zn deficiency (Supplementary Table 6). At that time, infants in Epoch-2 had less severe decrease in weight and length Z-scores from birth than those in Epoch-1 (Supplementary Table 6). In terms of growth patterns, fewer Zn-deficient patients were classified as having insufficient growth from birth to time of Zn level in Epoch-2 compared to Epoch-1 (49 vs 82%, P < 0.001), while more patients were classified as having insufficient weight gain during Epoch-2 vs Epoch-1 (17% vs 2%, P < 0.001) (Supplementary Table 6). Zn supplementation was provided to more infants in Epoch-2 than in Epoch-1 (13% vs 8%, P = 0.02) and started more frequently before 36 weeks’ PMA (P = 0.04) (Table 5). Thus, the frequency of diagnosis and treatment of Zn deficiency increased by > 30% in Epoch-2 and both occurred at lower PMA in Epoch-2.
Vitamin D
Serum 25-OH Vitamin D concentrations were measured at similar frequency at an average of 34–35 weeks’ PMA in both epochs (Table 5). Vitamin D deficiency and/or insufficiency was diagnosed more often in Epoch-2 vs in Epoch-1 (12% vs 7%, P = 0.03). Additionally, a larger percentage of those measured were found to be deficient/insufficient in Epoch-2 vs Epoch-1 (57% vs 38%, P = 0.008). As with Zn deficiency, vitamin D deficiency was associated with lower GA and with later introduction of formula if formula was not given (Supplementary Table 5).
Mortality and NEC versus DBM
Supplementary Table 7 summarizes NEC and death frequency observed in infants who did not receive DBM vs those who did. We observed higher mortality in infants who did not receive DBM when compared to infants who did receive DBM (11.1% vs 3.2%, P < 0.001). Frequency of NEC Stage ≥II did not differ based on administration of DBM (P = 0.99). On the other hand, death or surgical NEC was more frequent among infants who did not receive DBM (12.9% vs 4.9%, P < 0.001).
The adjusted odds of mortality were lower with increasing GA (P < 0.001) and increasing Apgar score at 5 min (P = 0.001), with administration of antenatal steroids (P = 0.003), prolonged duration of MOM and delayed introduction of formula (P < 0.001) (Supplementary Table 8). Adjusted odds of mortality were higher in small for gestational age (SGA) infants (P = 0.004), those with major congenital anomalies (P < 0.001), and in those who developed NEC stage III (P < 0.001).
Supplementary Table 8 summarizes the analysis of factors associated with death or NEC stage III. The adjusted odds of death or NEC stage III were lower with increasing GA (P < 0.001) and Apgar score at 5 min (P = 0.005), prolonged duration of MOM (P < 0.001) and delayed introduction of formula (P < 0.001), while they increased with major congenital anomalies (P < 0.001), sepsis (P < 0.001) and invasive mechanical ventilation (P = 0.04).
Discussion
DBM implementation did not significantly affect average change in weight, length or FOC growth from birth to discharge among infants receiving an adjustable feeding protocol with adjustment of energy, protein and micronutrients intake based on recommended intake for GA and age, growth rate and serial measurements of nutrients in the serum. However, the average protein and energy intake increased by 5% and the frequency of zinc and vitamin D supplementation increased by > 30% in Epoch-2. There was an increased frequency of infants with transient insufficient growth and vitamin D and Zn deficiency, and these were detected and corrected earlier. Implementation of the DBM did not decrease the length of time infants received MOM or the percentage of infants receiving MOM at discharge.
The frequency of diagnosis and treatment of Zn deficiency increased in Epoch-2. Since serum Zn concentration was obtained only for clinical indication, the total frequency of Zn deficiency in the cohort is not known. Since the frequency of obtaining serum Zn concentration did not increase in Epoch-2, increased frequency of diagnosis of Zn deficiency in Epoch-2 may have resulted from low Zn content of DBM compared with preterm formula [20, 21]. Early correction of Zn and/or vitamin D deficiencies, in addition to increased macronutrient content, may have led to correction of insufficient growth. This may explain why we did not observe a decrease in the Delta Z-scores of weight, length and FOC from birth to discharge in Epoch-2. The design of the study did not allow to differentiate the roles of macro- and micro-nutrients. To our knowledge, no study has evaluated the link between DBM feeding, micronutrient status and growth in VLBW infants.
In terms of major morbidities, we were particularly interested in how NEC frequency was affected by DBM implementation. We did not observe a significant decrease in the rate of NEC Stage ≥II during Epoch-2; however, the percentage of death and the percentage of death or NEC stage III were lower among infants who received DBM. Analysis of factors associated with this reduction shows that a later introduction of formula is associated with lower adjusted odds of these outcomes.
Some of this study’s strengths include that it examined a large sample and assessed growth in several ways. We examined changes in Z-scores from birth to discharge, but growth patterns may give more information on the timing and response of growth to feeding type and supplementation. This study also illustrates inaccuracy of length tape measurements.
Limitations of this study include a single center study, retrospective study design, lack of measuring MOM and DBM energy and protein concentration in each sample, using published average protein content at 4 weeks of life in maternal own milk for data during the entire NICU stay, limitation of growth studies to patients who survived to discharge (which might introduce some selection bias), and some subjectivity when assessing growth patterns. Additionally, Zn and Vitamin D concentrations were only measured when clinically indicated and as such are not available for the whole cohort. Therefore, the exact frequency of Zn deficiency and vitamin D insufficiency/deficiency in both epochs is not known, and the increase in frequency of diagnosis of micronutrient deficiency in Epoch-2 may have been biased by higher awareness of the link between poor growth and micronutrient deficiencies. Since only few infants had measurements of other micronutrients, it is possible that other deficiencies may not have been detected. Finally, accurate measurements with stadiometer were not available for every infant in the cohort.
Studies using propensity score analysis or dose-dependent analysis of MOM intake have shown neurodevelopmental benefits of MOM in VLBW and or very preterm infants [8]. Slower growth of DBM-fed preterm infants might negatively affect neurodevelopment [12,13,14]. However, growth rate and Bayley scores of infants randomized to DBM were similar to those randomized to formula in the DOMINO trial [42]. Since Zn deficiency has been associated with low Bayley scores [43], it is possible that early detection and supplementation of Zn deficiency in this study may improve neurodevelopment. Extremely low GA neonates in this study are being followed for growth and neurodevelopment at the THRIVE Clinic at Children’s Medical Center.
In conclusion, implementing a DBM program was not associated with a change in weight, length and FOC growth from birth to discharge in our NICU using an established adjustable feeding protocol. There was an increased frequency of infants with transient insufficient growth and vitamin D and Zn deficiency, and these were detected and corrected earlier. Close monitoring of growth patterns and awareness of possible nutritional deficiencies throughout the patient’s hospital stay allows for delay in formula through appropriate use of DBM feeding without compromising growth.
Data availability
The datasets generated and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Breastfeeding SO, Eidelman AI, Schanler RJ, Johnston M, Landers S, Noble L, et al. Breastfeeding and the Use of Human Milk. Pediatrics 2012;129:e827–e41.
Boquien CY. Human milk: An ideal food for nutrition of preterm newborn. Front Pediatr. 2018;6:295.
Lapidaire W, Lucas A, Clayden JD, Clark C, Fewtrell MS. Human milk feeding and cognitive outcome in preterm infants: the role of infection and NEC reduction. Pediatr Res. 2022;91:1207–14.
Brown JVE, Walsh V, McGuire W. Formula versus maternal breast milk for feeding preterm or low birth weight infants. Cochrane Database Syst Rev. 2019;8:Cd002972.
Strobel NA, Adams C, McAullay DR, Edmond KM. Mother’s own milk compared with formula milk for feeding preterm or low birth weight infants: systematic review and meta-analysis. Pediatrics 2022;150:e2022057092D.
Taylor SN, Fenton TR, Groh-Wargo S, Gura K, Martin CR, Griffin IJ, et al. Exclusive maternal milk compared with exclusive formula on growth and health outcomes in very-low-birthweight preterm infants: Phase II of the Pre-B project and an evidence analysis center systematic review. Front Pediatr. 2021;9:793311.
Miller J, Tonkin E, Damarell RA, McPhee AJ, Suganuma M, Suganuma H, et al. A systematic review and meta-analysis of human milk feeding and morbidity in very low birth weight infants. Nutrients 2018;10:707.
Peng W, Han J, Li S, Zhang L, Yang C, Guo J, et al. The association of human milk feeding with short-term health outcomes among chinese very/extremely low birth weight infants. J Hum Lact. 2022;38:670–7.
Underwood MA. Human milk for the premature infant. Pediatr Clin North Am. 2013;60:189–207.
Boundy EO, Anstey EH, Nelson JM. Donor human milk use in advanced neonatal care units - United States, 2020. MMWR Morb Mortal Wkly Rep. 2022;71:1037–41.
Nutrition CO, Breastfeeding SO, Fetus CO, Newborn, daniels S, Corkins M, et al. Donor human milk for the high-risk infant: Preparation, safety, and usage options in the United States. Pediatrics 2017;139:e20163440.
Quigley M, Embleton ND, McGuire W. Formula versus donor breast milk for feeding preterm or low birth weight infants. Cochrane Database Syst Rev. 2018;6:Cd002971.
Madore LS, Bora S, Erdei C, Jumani T, Dengos AR, Sen S. Effects of donor breastmilk feeding on growth and early neurodevelopmental outcomes in preterm infants: An observational study. Clin Ther. 2017;39:1210–20.
Montjaux-Régis N, Cristini C, Arnaud C, Glorieux I, Vanpee M, Casper C. Improved growth of preterm infants receiving mother’s own raw milk compared with pasteurized donor milk. Acta Paediatr. 2011;100:1548–54.
Parker LA, Cacho N, Engelmann C, Benedict J, Wymer S, Michael W, et al. Consumption of mother’s own milk by infants born extremely preterm following implementation of a donor human milk program: A retrospective cohort study. J Pediatr. 2019;211:33–8.
Fu TT, Schroder PE, Poindexter BB. Macronutrient analysis of target-pooled donor breast milk and corresponding growth in very low birth weight infants. Nutrients 2019;11:1884.
Gidrewicz DA, Fenton TR. A systematic review and meta-analysis of the nutrient content of preterm and term breast milk. BMC Pediatr. 2014;14:216.
Fu TT, Kaplan HC, Fields T, Folger AT, Gordon K, Poindexter BB. Protein enrichment of donor breast milk and impact on growth in very low birth weight infants. Nutrients 2021;13:2869.
Agostoni C, Buonocore G, Carnielli V, De Curtis M, Darmaun D, Decsi T, et al. Enteral nutrient supply for preterm infants: Commentary from the European society of paediatric gastroenterology, hepatology and nutrition committee on nutrition. J Pediatr Gastroenterol Nutr. 2010;50:85–91.
Gates A, Marin T, Leo G, Stansfield BK. Review of preterm human-milk nutrient composition. Nutr Clin Pr. 2021;36:1163–72.
Young BE, Borman LL, Heinrich R, Long J, Pinney S, Westcott J, et al. Effect of pooling practices and time postpartum of milk donations on the energy, macronutrient, and zinc concentrations of resultant donor human milk pools. J Pediatr. 2019;214:54–9.
Alshaikh B, Abo Zeed M, Yusuf K, Guin M, Fenton T. Effect of enteral zinc supplementation on growth and neurodevelopment of preterm infants: A systematic review and meta-analysis. J Perinatol. 2022;42:430–9.
Brion LP, Heyne R, Lair CS. Role of zinc in neonatal growth and brain growth: Review and scoping review. Pediatr Res. 2021;89:1627–40.
Staub E, Evers K, Askie LM. Enteral zinc supplementation for prevention of morbidity and mortality in preterm neonates. Cochrane Database Syst Rev. 2021;3:Cd012797.
Gomes FP, Shaw PN, Whitfield K, Koorts P, McConachy H, Hewavitharana AK. Effect of pasteurisation on the concentrations of vitamin D compounds in donor breastmilk. Int J Food Sci Nutr. 2016;67:16–9.
Gates A, Marin T, De Leo G, Waller JL, Stansfield BK. Nutrient composition of preterm mother’s milk and factors that influence nutrient content. Am J Clin Nutr. 2021;114:1719–28.
við Streym S, Højskov CS, Møller UK, Heickendorff L, Vestergaard P, Mosekilde L, et al. Vitamin D content in human breast milk: a 9-mo follow-up study. Am J Clin Nutr. 2016;103:107–14.
Casey CF, Slawson DC, Neal LR. Vitamin D supplementation in infants, children, and adolescents. Am Fam Physician. 2010;81:745–8.
Yang Y, Li Z, Yan G, Jie Q, Rui C. Effect of different doses of vitamin D supplementation on preterm infants - an updated meta-analysis. J Matern Fetal Neonatal Med. 2018;31:3065–74.
Ma K, Wei SQ, Bi WG, Weiler HA, Wen SW. Effect of Vitamin D supplementation in early life on children’s growth and body composition: A systematic review and meta-analysis of randomized controlled trials. Nutrients 2021;13:524.
Mølgaard C, Michaelsen KF. Vitamin D and bone health in early life. Proc Nutr Soc. 2003;62:823–8.
Pavageau L, Rosenfeld CR, Heyne R, Brown LS, Whitham J, Lair C, et al. Valid serial length measurements in preterm infants permit characterization of growth patterns. J Perinatol. 2018;38:1694–701.
Brion LP, Rosenfeld CR, Heyne R, Brown SL, Lair CS, Burchfield PJ, et al. Adjustable feedings plus accurate serial length measurements decrease discharge weight-length disproportion in very preterm infants: quality improvement project. J Perinatol. 2019;39:1131–9.
Mandiá N, Bermejo-Barrera P, Herbello P, López-Suárez O, Fraga JM, Fernández-Pérez C, et al. Human milk concentrations of minerals, essential and toxic trace elements and association with selective medical, social, demographic and environmental factors. Nutrients. 2021;13:1885.
Domellöf M. Nutritional care of premature infants: Microminerals. World Rev Nutr Diet. 2014;110:121–39.
Sanchez AM, Mize SG, Jimenez JM, Manroe BL, Rosenfeld CR, Tyson TE. Systems approach to the evaluation of maternal and neonatal care. Proc 12th Hawaii Int’l Conf Syst Sci, Sel Pap Med Inf Process. 1979;III:140–51.
Kaiser JR, Tilford JM, Simpson PM, Salhab WA, Rosenfeld CR. Hospital survival of very-low-birth-weight neonates from 1977 to 2000. J Perinatol. 2004;24:343–50.
Kliegman, RM, Walsh, MC. Neonatal necrotizing enterocolitis: Pathogenesis classification and spectrum of illness. Current Problems in Pediatrics. 1987;17:219–88. https://doi.org/10.1016/0045-9380(87)90031-4.
John A, Sun R, Maillart L, Schaefer A, Hamilton Spence E, Perrin MT. Macronutrient variability in human milk from donors to a milk bank: Implications for feeding preterm infants. PLoS One. 2019;14:e0210610.
Rochow N, Raja P, Liu K, Fenton T, Landau-Crangle E, Göttler S, et al. Physiological adjustment to postnatal growth trajectories in healthy preterm infants. Pediatr Res. 2016;79:870–9.
Brion LP, Heyne R, Brown LS, Lair CS, Edwards A, Burchfield PJ, et al. Zinc deficiency limiting head growth to discharge in extremely low gestational age infants with insufficient linear growth: a cohort study. J Perinatol. 2020;40:1694–704.
O’Connor DL, Gibbins S, Kiss A, Bando N, Brennan-Donnan J, Ng E, et al. GTA DoMINO feeding group. Effect of supplemental donor human milk compared with preterm formula on neurodevelopment of very low-birth-weight infants at 18 months: A randomized clinical trial. JAMA 2016;316:1897–905.
Boscarino G, Gasparini C, Conti MG, Di Chiara M, Terrin G. Zinc levels in neonatal life influence long-term neurodevelopment. J Perinatol. 2021;41:1196–7.
Kim SY, Park JH, Kim EA, Lee-Kim YC. Longitudinal study on trace mineral compositions (selenium, zinc, copper, manganese) in Korean human preterm milk. J Korean Med Sci. 2012;27:532–6.
Casey CE, Neville MC, Hambidge KM. Studies in human lactation: Secretion of zinc, copper, and manganese in human milk. Am J Clin Nutr. 1989;49:773–85.
Acknowledgements
This study was funded by the Children’s Medical Center Clinical Advisory Committee (CCRAC) (LP Brion) and by the Department of Pediatrics at UT Southwestern Medical Center.
Author contributors
MSR conceptualized and designed the study, collected and reviewed data from the medical records and wrote the first draft of the manuscript. CL, AE, and TJ collected and reviewed data from the medical records, participated in the interpretation of the data. LPB conceptualized and designed the study, collected and reviewed data from the medical records. LPB and LSB conducted statistical analyses. PJB, PS, IK, DV, and CC, IT specialist at Parkland Hospital, extracted data from the electronic health record. All authors participated in the interpretation of the data, critically reviewed the revisions, and approved the final manuscript as submitted. We thank Mambarambath Jaleel, MD and Elen Petrosyan, RD, who were instrumental in implementing the DBM program. Aktar Ali, MS performed DBM sample analysis for copper and zinc in Dr. Orson Moe’s laboratory at UT Southwestern Medical Center. Anita Thomas, RN, helped with data collection into the resuscitation database.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Sanchez-Rosado, M., Lair, C.S., Edwards, A. et al. Growth after implementing a donor breast milk program in neonates <33 weeks gestational age or birthweight <1500 grams: Retrospective cohort study. J Perinatol 43, 608–615 (2023). https://doi.org/10.1038/s41372-023-01627-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41372-023-01627-2