Abstract
Objective
We evaluated first screen pass rate for two pass thresholds for critical congenital heart disease (CCHD) oxygen saturation (SpO2) screening at higher altitude.
Study design
A retrospective cohort of 948 newborns underwent CCHD screening near sea-level (n = 463) vs 6250 ft altitude (n = 485) over 3 years. Standard SpO2 pass threshold ≥95% and lower SpO2 pass threshold ≥93% (high-altitude screen) were applied to first measurements to compare pass frequencies.
Results
The median SpO2 was lower in high-altitude newborns (96% vs 99%—p < 0.001). The high-altitude newborns passed the AAP algorithm first screen less often (89.3% vs 99.6%—p < 0.001). With the high-altitude algorithm, 98% of high-altitude newborns passed the first screen.
Conclusion
Lowering the SpO2 pass threshold by 2% at >6000 ft, significantly increased first screen pass rate. Adjustments for altitude may reduce nursing time to conduct repeat measurements and prevent transfers for echocardiograms. Larger studies are necessary to assess impact on false negatives.
Similar content being viewed by others
Introduction
Critical congenital heart disease (CCHD) screening using pulse oximetry was recommended by the US Health and Human Services Secretary’s Advisory Committee on Heritable Disorders in Newborns and Children and was added to the recommended uniform screening panel [1]. The American Academy of Pediatrics (AAP) workgroup provided an algorithm for universal screening of newborns [2]. A Cochrane review of CCHD screening using similar oxygen saturation (SpO2) thresholds as the recommended AAP algorithm showed a low false positive rate of 0.14%. The false positive rate was lower (0.06%) when the screen was completed after 24 h after birth [3]. However, few studies have evaluated the performance of the screening at high altitude (>6000 ft) [4, 5]. The American Academy of Pediatrics (AAP) recommends that “algorithm cutoffs may need to be adjusted in high-altitude nurseries.” [2] However, no specific approach is specified for high-altitude CCHD screening.
More recently, studies have recognized that modifications to this algorithm are needed at high altitude to reduce the frequency of screening failures [6, 7]. Barton Memorial Hospital in South Lake Tahoe, California (elevation > 6000 ft) implemented a modified algorithm using a threshold of ≥93% (instead of ≥95% in the AAP algorithm) as their criterion to pass CCHD screening without prompting repeat measurements due to their initial experience with false positives with a higher threshold. We hypothesized, based on physiologic data (Table 1), this threshold at ≥93% for a passing screen at higher altitude would result in higher first pass screen compared to the standard threshold of ≥95% without need to prompt repeat measurement.
Methods
This was a retrospective cohort review of newborns undergoing routine SpO2-based CCHD screening at two altitudes in Northern California. University of California, Davis in Sacramento was the near-sea level site (30 ft elevation from sea level) and Barton Memorial Hospital in South Lake Tahoe, California was the high-altitude site (6250 ft elevation). University of California, Davis Institutional Review Board approved this study for both sites.
We estimated that 3 years of data would be necessary for adequate sample size from the high-altitude site (see analysis for sample size). Thus, we included patients of the same time period from the lower altitude site, January 2016 to December 2018. Due to higher birth rate at the lower altitude site, we included select patients to result in similar number of patients at each site. To select patients from the lower altitude site, we sorted by alphabetical order and then selected every 7th patient to ensure there was not a chronological pattern to the selection.
Protocols for CCHD screening were standardized at each site during these time periods. Patients that underwent routine SpO2 CCHD screening were included. Both sites performed SpO2 CCHD screening after 24 h of age or just before discharge if a newborn was discharged before 24 h. Subjects were excluded if they were admitted to the Neonatal Intensive Care Unit (NICU), transferred, or had an echocardiogram completed before completion of the routine SpO2 CCHD screening. Patients that were admitted to the NICU, transferred or had echocardiogram completed following the routine SpO2 CCHD screen were included since the results of the screen could lead to these interventions. They were also excluded if SpO2 screening results with numeric values were not available. Electronic medical records were reviewed for SpO2 screening results (interpretation and SpO2 values), medical conditions, echocardiograms and procedures. To identify potentially false negative screens, follow up encounters within the medical system, at least 6 weeks after birth, were reviewed for evidence of cardiac disease.
The SpO2 CCHD screening protocol at the near-sea level site during the studied period followed the AAP algorithm as outlined by Kemper et al. and consistent with the algorithm provided in the California Department of Health Care Services (DHCS) guidance provided in 2016 [2, 8]. In this algorithm, the SpO2 measurement was considered failing if (1) any SpO2 measurement was <90%, or (2) SpO2 90 to <95% in both right hand and foot and/or a >3% absolute difference between the right hand and foot on three measurements. Any SpO2 measurement ≥95% in either the right hand or foot with ≤3% absolute difference was considered passing [2]. The SpO2 CCHD screening protocol at the high altitude site during the studied period used a lower SpO2 threshold (Fig. 1). In the high-altitude algorithm, the SpO2 measurement was considered failing if <93% in both right hand and foot or a >3% absolute difference between the right hand and foot on three measurements. Any SpO2 measurement ≥93% in either the right hand or foot with ≤3% absolute difference was considered passing. In this high-altitude algorithm, an SpO2 ≤ 90% prompts a physician assessment who then considers echocardiogram if other etiologies for the hypoxemia are not determined, which is similar to the AAP-Kemper algorithm [2]. In the high-altitude algorithm, the physician may recommend repeat SpO2 testing after the physician assessment for an SpO2 ≤ 90%, which is different than the AAP-Kemper algorithm.
Modified algorithm allows for physician to determine if an echocardiogram should be obtained for oxygen saturation ≤90% before repeating the screen. However, the physician must be notified for the oxygen saturation ≤90% whereas an oxygen saturation 91–92% triggers repeat screening. RH right hand, F foot.
Statistical analysis
Our primary outcome was first-time SpO2 CCHD screen pass rate. We evaluated this pass rate for each sites’ screening protocol. We also evaluated the first-time pass rate for babies at high altitude using the AAP-Kemper SpO2 pass threshold of ≥95%. We estimated 381 newborns in each group would provide power 0.9 with alpha 0.05 to detect an increase in initial screen pass rate from 95% to 99.9%. We suspected ~60–75% of patients would have documented follow up within their birth hospital system and that not all newborns would have documented SpO2 numeric values. Thus, we targeted ~500 newborns in each group. Summary statistics for the newborns were presented as medians or frequencies with interquartile ranges (IQRs) or percentages, respectively. The medians of continuous data were compared using the nonparametric equality-of-medians test. The Pearson chi-square or Fisher exact test, as appropriate, was used to compare categorical data. A p value ≤ 0.05 was considered statistically significant. The data were analyzed with Stata Statistical Software, release 15.1 (Stata Corp, College Station, TX).
Results
The final cohort of newborns included 463 newborns near sea level and 485 at high altitude. Demographic characteristics for the cohort are presented in Table 2. The newborns near sea level were less likely to be white (48% vs 90%, p < 0.001) compared to the newborns at high altitude. The newborns near sea level were more likely born via cesarean section (31% vs 24%, p = 0.02) compared to newborns at high altitude. The sea level newborns also had higher frequency of family history of congenital heart disease (4% vs 1%, p = 0.008).
Medical conditions between the two groups did not differ with the exception of neonatal respiratory conditions, which were more common in the newborns at high altitude (2% near sea level vs 8% high altitude, p < 0.001) (Table 2). Neonatal respiratory conditions were combined to include persistent pulmonary hypertension, transient tachypnea of newborn, respiratory distress syndrome, lung malformation, pneumothorax, meconium aspiration, and sepsis. The most common neonatal respiratory illness was transient tachypnea of newborn for both groups (80% of the near sea level newborns and 92% of the high-altitude newborns with respiratory illnesses). Respiratory illnesses that presented prior to the newborn undergoing routine SpO2 CCHD screen were not evaluated as these newborns were excluded. The two groups were admitted to the NICU at similar rates (1% in both). Only one patient at high altitude was transferred after their routine CCHD screen, which they passed. This patient had transient tachypnea of the newborn requiring continuous positive pressure prompting the transfer.
Oxygen saturation CCHD screen results
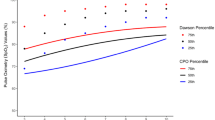
When applying the AAP-Kemper algorithm to all patients, high altitude patients were less likely to pass on the first SpO2 measurement (99.6% vs 89.3%) and more likely to require repeat screening (0.4% vs 10.1%) compared to newborns near sea level (p < 0.001). The adjusted high altitude SpO2 threshold (≥93% as opposed to ≥95% as a passing screening) resulted in 98% (N = 475) passing, 1% (N = 5) requiring repeat screening, and 1% (N = 5) requiring notifying the physician after their first SpO2 screen in the high-altitude newborns. The median preductal and postductal SpO2 from the first CCHD screen were lower in the high-altitude newborns (Table 3). We also evaluated how often either the first preductal or postductal SpO2 measurement was <95%. First SpO2 measurements were more likely to be <95% in the high-altitude newborns compared to newborns near sea level for both the preductal (21% vs 0.4% respectively, p < 0.001) and postductal (20% vs 0 respectively, p < 0.001) measurements.
Confirmation of cardiac disease
To identify potentially false negative screens, follow up encounters within the medical system, at least 6 weeks after birth, were reviewed for evidence of cardiac disease. Examinations at 6 weeks of age or later were noted in the medical record for 66% of patients near sea level and 88% of high-altitude patients. No evidence of false negative screens (defined as evidence of CCHD in a newborn that passed SpO2 screening) was found in either group. The two groups had echocardiograms completed at similar frequencies, 2.8% (N = 13) of patients near sea level and 1.9% (N = 9) of high-altitude patients (p = 0.3). Of the patients that had echocardiograms, 56% of high-altitude patients (5 of 9) had abnormal echocardiograms while 23% of patients at sea level (3 of 13). The differences in abnormal echocardiograms were not significant (p = 0.12). Patent ductus arteriosus and/or patent foramen ovale were not considered abnormal. The echocardiogram abnormalities were ventricular septal defects (N = 7), and mild pulmonary hypertension (N = 1). We performed a secondary analysis restricting our patient population to those with a documented follow up examination at 6 week of age or older. Even after this restriction, the high-altitude patients were still more likely to require repeat SpO2 measurement after the first measurement compared to patients near sea level (0.3% vs 11%, p < 0.001) when using the AAP-Kemper algorithm. None of the infants included in the study underwent cardiac catheterization or cardiac surgery at the Regional Perinatal Center in the first month after birth.
Discussion
Lowering the SpO2 CCHD passing threshold to ≥93% increases the frequency of first screen pass among newborns at high altitude (>6000 ft). When using a SpO2 threshold ≥95%, less than 90% of newborns at high altitude passed their first screen. Decreasing the pass threshold to ≥93% resulted in 98% of newborns at high altitude passing on the first CCHD screen. This is not surprising considering the median preductal and postductal SpO2 results were 96% in the high-altitude patients in our cohort. We obtained follow-up data for 88% of the high-altitude patients after at least 6 weeks of age and did not find evidence of a missed CCHD in a patient that passed the SpO2 screen, or in other words, we did not find evidence of a false negative screen.
The SpO2 thresholds implemented in initial AAP-recommended CCHD screening algorithm were based on studies of thousands of newborns, including newborns both with and without CCHD [2, 9, 10]. Since then, universal SpO2 screening has improved early detection of CCHD and decreased mortality [11]. SpO2-based CCHD screening has also been noted to have a small false positive rate at 0.14% overall and 0.06% if performed 24 h after birth or later [3]. However, it is notable that the recommended SpO2 thresholds were based on studies on newborns predominantly at lower altitude [9, 10]. Furthermore, ways to further improve the algorithm, including at higher altitude, have been noted [12]. Hospitals at high altitude have noted increased false positive rates using the standard AAP SpO2 thresholds leading to a significant increase in the number of unnecessary echocardiograms required [4, 5]. Considering the most recent updated CCHD algorithm now only requires one repeat measurement as opposed to two before classifying as a failed screen and potentially triggering an echocardiogram, the pass threshold at high altitude is even more crucial to clarify [12]. In theory, in this 2020 recommended algorithm, using the standard pass threshold at higher altitude could result in a larger overall screen fail rate as the newborns would have fewer opportunities to pass the screen.
In Table 4, we provided a summary of prior studies that made adjustments to the screening algorithm at higher altitude and will discuss some of them further here [4, 5, 13, 14]. Some centers at high altitude, such as Aurora CO, have altered their algorithms by using a cut-off of <85% for a positive screen (instead of <90% in the AAP algorithm) and ≥90% with <3% preductal postductal difference on repeated attempts as a screen negative (instead of ≥95% in the AAP algorithm) [4]. However, in this algorithm first screens still require ≥95% in the preductal or postductal SpO2 on the initial screens to pass without needing to repeat the measurement [4]. In another modified high altitude algorithm, the initial and overall SpO2 passing thresholds also remain ≥95% [14]. For example, some hospitals in Colorado have made modifications such as delaying the screen to 30 h after birth to allow further transitioning, lowering the SpO2 failure threshold to <85%, and trialing oxygen hood to increase PIO2 for 20 min for those requiring repeat screens [4, 6, 14]. These algorithms however still require SpO2 ≥ 95% to pass the screen. Therefore, these adjustments may lower the overall false positive rate as noted by refs. [4, 14] (Table 4). However, they may still require additional nursing time to repeat screening measurements. The non-passing rate of the first screen in these studies was 5.8% and 3.6%, much higher than the non-passing rate of newborns at sea level or newborns at high altitude with the threshold of ≤93% in our cohort (2%). The modified high-altitude screening thresholds described in our study allowed for a significant reduction in the number of patients requiring repeat screening measurements from 10.1% to 2%.
Our first non-pass rates when using the AAP threshold ≥95% are higher than some prior studies. For example, 10.1% of our high-altitude patients did not pass the first screen with this standard threshold, which is higher than the non-pass rates reported by Wright et al. and Lueth et al. at similar altitude ~5000–6000 ft [4, 14]. Our higher non-pass on the first screen is likely due to our retrospective design versus their prospective and possibly more controlled approach, or due to site-to-site variation. A multicenter study of various altitudes conducted by Paranka et al., also showed an increase in the positive screen rate with increasing altitude. However, when using the AAP-Kemper passing threshold of ≥95%, only 6% of newborns >6000 ft had a positive screen [5]. Our higher false positive rate compared to Paranka et al. findings are likely due to us only evaluating the first SpO2 measurement as opposed to the overall screening algorithm. If our patients had proceeded onto repeat measurements, then the overall positive screen rate presumably would have decreased.
Our findings are consistent with physiologic considerations at high altitude in newborn infants (Table 1). Increasing altitude reduces barometric pressure and PIO2. Calculations based on barometric pressure data demonstrate a 27 mmHg reduction in Alveolar PAO2 at 6000 ft altitude compared to sea level. The Alveolar to arterial gradient (A-a gradient) is higher in healthy newborn infants compared to adults and is reported to be 28.3 ± 10.1 mmHg [15]. However, in adult models, the A-a gradient decreases at high altitude and a similar mechanism can be expected in neonates resulting slightly higher PaO2 values than expected based on drop in alveolar PAO2 (Table 1) [16]. Presence of fetal hemoglobin and high respiratory rates, leading to alkalosis, shift the oxygen-hemoglobin curve to the left resulting in higher SpO2 for a given PaO2 [17, 18]. For these physiological reasons, neonates at high altitude tend to have relatively higher SpO2 despite low PaO2.
Interesting that despite difference in access to a NICU, the two sites had similar admission rate to the NICU (1%). The site near sea level has a level IV NICU within the same building whereas the high-altitude sites does not have a local NICU and the closest NICU is either a flight or drive on a mountainous road away. Thus, we expected that the well newborn nursery at the high-altitude site may manage higher acuity patients compared to the near sea level site to avoid a transfer. This may still be the case though, as we excluded patients that were transferred to the NICU prior to their routine SpO2 screen or if the SpO2 was measured prior to 24 h of age due to symptoms rather than early discharge, in order to isolate only the SpO2 measurements done purely for screening in asymptomatic infants. Despite difference in access to echocardiograms between the two sites, echocardiograms were also obtained at similar rates at the two sites despite differences in screen pass rates. Our approach was limited to review the indication for echocardiograms, but we suspect the majority were done due to other clinical indications such as findings of murmur on physical examination.
There are several limitations to our study. As a retrospective study with a total sample size of 948 patients, we are limited by the documentation in the chart and limited ability to estimate false negative screen rates. Due to the low incidence of CCHD we were unable to identify any CCHD cases in our population. This could also be due to the improved prenatal detection of CCHD cases that led to prompt NICU admissions at birth and exclusion from our study cohort. This is consistent with similar CCHD screening studies that were unable to identify cases of CCHD in their cohort [19,20,21]. Hence, we could not confirm the effect of the altered CCHD screening protocol on false negative rates. Additionally, we were unable to review birth defect registries for possible false negative cases as the California Birth Defects Monitoring Program Registry monitors ten counties (30% of births in California). The studied population was not in one of those monitored counties [22]. We were however able to confirm follow up and absence of concern for congenital heart disease in 88% of the patients at high altitude. Additionally, there are only two major cardiac centers within California that are near the high-altitude center, of which our near sea level site is one of them and due to partnership between the two sites it is most likely a patient would have been transferred to our center. We were also limited by the SpO2 values actually performed. Thus, when applying the AAP-Kemper algorithm to the patients at high altitude, we were not able to assess the overall false positive rate of the algorithm as the patients did not always have a repeated measurement that would have been triggered by that algorithm. None the less, our findings that an altered pass threshold reducing the need for repeat measurements is important. Additionally, in the context of recent recommendations that reduce the number of repeat screens before considering a screen as a failed screen, this should be evaluated further specifically at higher altitude.
In conclusion, lowering SpO2 CCHD pass threshold by just 2% (from ≥95% to ≥93%) at 6000 ft, significantly increased pass rate on first screen. It also does not appear to increase false negative screens; however, further studies with larger samples will be needed to identify missed CCHD cases and should evaluate the diagnosis of other diseases with hypoxemia as well (i.e., persistent pulmonary hypertension of the newborn). Adjusting the CCHD pass threshold may reduce nursing time associated with unnecessary repeat measurements and may reduce overall healthcare spending due to avoidable echocardiograms. It can also prevent unnecessary transfers to tertiary hospitals. Therefore, an altered screening protocol at high altitudes may reduce parental anxiety of a failed screen due to the additional hospital length of stay and the prohibitive costs of additional diagnostics. Our findings suggest altitudes 5001–7500 ft could consider reducing the threshold to ≥93% similar to our study. However, larger samples are needed to confirm these findings. Using larger samples, it might be possible to come up with a simple algorithm (such as reducing the threshold from 95% by 1% for every 1000 m above sea level). The risk of false negatives with modifications to the thresholds needs to be considered and studied as well. If the passing thresholds for CCHD screening do not change at higher altitude due to risk of false negatives, then other mitigation efforts such as improved local access to echocardiogram should be considered. We recommend that individual high-altitude centers evaluate their CCHD screening algorithm and publish their data to enable AAP and CDC to come up with new algorithms for high-altitude screening.
Data availability
The datasets generated and analyzed during this study are not publicly available through a repository but are available from the corresponding author on reasonable request and may require institutional data agreements.
References
Mahle WT, Martin GR, Beekman RH, Morrow WR. Endorsement of Health and Human Services recommendation for pulse oximetry screening for critical congenital heart disease. Pediatrics. 2012;129:190–2. https://doi.org/10.1542/peds.2011-3211
Kemper AR, Mahle WT, Martin GR, Cooley WC, Kumar P, Morrow WR, et al. Strategies for implementing screening for critical congenital heart disease. Pediatrics. 2011;128:e1259–67. https://doi.org/10.1542/peds.2011-1317
Plana M, Zamora J, Suresh G, Thangaratinam S, Ewer A. Pulse oximetry screening for critical congenital heart defects (Review). Cochrane Database Syst Rev. 2018:1–38. https://doi.org/10.1002/14651858.CD011912.pub2. www.cochranelibrary.com
Wright J, Kohn M, Niermeyer S, Rausch CM. Feasibility of critical congenital heart disease newborn screening at moderate altitude. Pediatrics. 2014;133:e561–9. https://doi.org/10.1542/peds.2013-3284
Paranka MS, Brown JM, White RD, Park MV, Kelleher AS, Clark RH. The impact of altitude on screening for critical congenital heart disease. J Perinatol. 2018;38:530–6. https://doi.org/10.1038/s41372-018-0043-9
Oster ME, Aucott SW, Glidewell J, Hackell J, Kochilas L, Martin GR, et al. Lessons learned from newborn screening for critical congenital heart defects. Pediatrics. 2016;137:e20154573–e20154573. https://doi.org/10.1542/peds.2015-4573
Samuel TY, Bromiker R, Mimouni FB, Picard E, Lahav S, Mandel D, et al. Newborn oxygen saturation at mild altitude versus sea level: Implications for neonatal screening for critical congenital heart disease. Acta Paediatr Int J Paediatr. 2013;102:379–84. https://doi.org/10.1111/apa.12155
Assembly Bill 1731. Newborn Screening Program: Critical Congenital Heart Disease. California: General Assembly; 2012.
de-Wahl Granelli A, Wennergren M, Sandberg K, Mellander M, Bejlum C, Inganas L, et al. Impact of pulse oximetry screening on the detection of duct dependent congenital heart disease: a Swedish prospective screening study in 39 821 newborns. BMJ. 2008;337:a3037 https://doi.org/10.1136/bmj.a3037
Granelli AD-W, Mellander M, Sunnegårdh J, Sandberg K, Östman-smith I. Screening for duct-dependent congenital heart disease with pulse oximetry: a critical evaluation of strategies to maximize sensitivity. Acta Pædiatr. 2005;94:1590–6. https://doi.org/10.1111/J.1651-2227.2005.TB01834.X
Abouk R, Grosse S, Ailes E, Oster M. Association of US state implementation of newborn screening policies for critical congenital heart disease with early infant cardiac deaths. JAMA. 2017;318:2111–8. https://doi.org/10.1001/jama.2017.17627
Martin GR, Ewer AK, Gaviglio A, Hom LA, Saarinen A, Sontag M, et al. Updated strategies for pulse oximetry screening for critical congenital heart disease. Pediatrics. 2020;146:e20191650 https://doi.org/10.1542/peds.2019-1650
Rao S, Goens MB, Myers OB, Sebesta EA. Pulse oximetry screening for detection of congenital heart defects at 1646 m in Albuquerque, New Mexico. Cardiol Young-. 2020;30:1851–5. https://doi.org/10.1017/S1047951120002899
Lueth E, Russell L, Wright J, Duster M, Kohn M, Miller J, et al. A novel approach to critical congenital heart disease (CCHD) screening at moderate altitude. Int J Neonatal Screen. 2016;2:4 https://doi.org/10.3390/ijns2030004
Nelson NM, Prod’hom LS, Cherry RB, Lipsitz PJ, Smith CA. Pulmonary function in the newborn infant: the alveolar-arterial oxygen gradient. J Appl Physiol. 1963;18:534–8. https://doi.org/10.1152/jappl.1963.18.3.534
Sylvester JT, Cymerman A, Gurtner G, Hottenstein O, Cote M, Wolfe D. Components of alveolar-arterial O2 gradient during rest and exercise at sea level and high altitude. J Appl Physiol Respir Environ Exerc Physiol. 1981;50:1129–39. https://doi.org/10.1152/jappl.1981.50.6.1129
Lakshminrusimha S, Manja V, Mathew B, Suresh GK. Oxygen targeting in preterm infants: a physiological interpretation. J Perinatol. 2015;35:8–15. https://doi.org/10.1038/jp.2014.199
Crocker ME, Hossen S, Goodman D, Simkovich SM, Kirby M, Thompson LM, et al. Effects of high altitude on respiratory rate and oxygen saturation reference values in healthy infants and children younger than 2 years in four countries: a cross-sectional study. Lancet Glob Heal. 2020;8:e362–73. https://doi.org/10.1016/S2214-109X(19)30543-1
Riede FT, Wörner C, Dähnert I, Möckel A, Kostelka M, Schneider P. Effectiveness of neonatal pulse oximetry screening for detection of critical congenital heart disease in daily clinical routine-results from a prospective multicenter study. Eur J Pediatr. 2010;169:975–81. https://doi.org/10.1007/s00431-010-1160-4
Garg LF, Van Naarden Braun K, Knapp MM, Anderson TM, Koppel RI, Hirsch D, et al. Results from the New Jersey statewide critical congenital heart defects screening program. Pediatrics. 2013;132:e314–23. https://doi.org/10.1542/peds.2013-0269
Johnson LC, Lieberman E, O’Leary E, Geggel RL. Prenatal and newborn screening for critical congenital heart disease: findings from a nursery. Pediatrics. 2014;134:916–22. https://doi.org/10.1542/PEDS.2014-1461
Health CD of P. California Birth Defects Monitoring 2021. https://www.cdph.ca.gov/Programs/CFH/DGDS/Pages/cbdmp/HeartDefects.aspx
Martin RJ, Okken A, Rubin D. Arterial oxygen tension during active and quiet sleep in the normal neonate. J Pediatr. 1979;94:271–4. https://doi.org/10.1016/S0022-3476(79)80842-2
Jegatheesan P, Song D, Angell C, Devarajan K, Govindaswami B. Oxygen saturation nomogram in newborns screened for critical congenital heart disease. Pediatrics. 2013;131. https://doi.org/10.1542/peds.2012-3320
Ravert P, Detwiler T, Dickinson K. Mean oxygen saturation in well neonates at altitudes between 4498 and 8150 feet. Adv Neonatal Care. 2011;11:412–7.
Acknowledgements
We would like to thank Nicki Naylor and Eric Chavez from Barton Memorial Hospital for their assistance with data extraction.
Funding
The project described was supported by the National Center for Advancing Translational Sciences, National Institutes of Health (NIH), through grant number UL1 TR001860 and linked award KL2 TR001859, the Eunice Kennedy Shriver National Institute of Child Health & Human Development, NIH, through grant number 1R21HD099239 and 5R01HD072929. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Author information
Authors and Affiliations
Contributions
HS conceptualized and designed the study, completed the analysis, drafted the initial manuscript, and reviewed and revised the manuscript. MRS, PV, and SL conceptualized and designed the study, interpreted the data analysis, and critically reviewed and revised the manuscript.
Corresponding author
Ethics declarations
Competing interests
HS and SL are listed as inventors on a patent filed by UC Davis regarding a machine learning algorithm that combines pulse oximetry features for CCHD screening. HS is the founding member of NeoPOSE, a company aimed at developing technology to improve CCHD detection.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Sneeringer, M.R., Vadlaputi, P., Lakshminrusimha, S. et al. Lower pass threshold (≥93%) for critical congenital heart disease screening at high altitude prevents repeat screening and reduces false positives. J Perinatol 42, 1176–1182 (2022). https://doi.org/10.1038/s41372-022-01491-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41372-022-01491-6
This article is cited by
-
A new algorithm DEtectS critical Congenital Heart Disease at different altitudes: ANDES-CHD study
Journal of Perinatology (2024)
-
Trajectories of oxygen saturation within 6–72 hours after birth in neonates at moderate altitude: a prospective longitudinal cohort study
World Journal of Pediatrics (2023)