Abstract
Objectives
To compare survival and short-term respiratory outcomes of infants weighing <750 g initially intubated with 2.0 mm versus 2.5 mm endotracheal tube (ETT).
Study design
Retrospective, observational cohort study.
Results
Of 149 inborn infants weighing <750 g admitted to the NICU, 69 (46%) were intubated with 2.0 mm ETT, 78 with 2.5 mm ETT (53%), and 2 infants never required intubation. Infants intubated with 2.0 mm ETT were more premature (median gestational age (GA) 23 weeks (22, 24) vs. 24 weeks (24, 25) p < 0.0001), smaller (median birth weight 545 g (450, 616) vs. 648 g (579, 700), p < 0.0001), and more frequently intubated at delivery (96% vs. 68%, p < 0.00001). Survival to discharge was similar 77%, 53/69 and 87%, 68/78 (p = 0.09). Adjusted for GA, there were no significant differences in ventilator days (p = 0.7338) or Grade 3 BPD.
Conclusions
Premature infants born at a median GA of 23 weeks and median birth weight of 545 g can be successfully managed with 2.0 mm ETT.
Similar content being viewed by others
Introduction
Approximately 0.5% of all births in the United States occur at less than 28 weeks of gestation [1] and the rate of infants born with very low birth weight has increased from 1.2% in 1983 to 1.5% in 2005 [2]. The survival of extremely premature infants has improved over the past two decades, with improvements in both obstetrical and neonatal medicine [3, 4]. Advances in neonatal ventilation strategies have significantly contributed to the increased survival of extremely preterm infants due to their dependence on respiratory support [5].
Despite these advances in medical care and improvement in survival, extremely preterm and low birth-weight infants continue to have significant morbidity, mortality, and long-term neurodevelopmental disability. For these reasons, the decision to resuscitate an infant near the limit of viability (22–24 weeks of gestation) is extremely difficult. Birth weight and gestational age are thought to be strong predictors of outcomes and therefore these two parameters are often used to guide decision making regarding the initiation of neonatal intensive care, but female sex, prenatal corticosteroid treatment, and single vs multiple births have also been shown to be predictors of favorable outcomes [6,7,8]. In some centers, a mechanical rather than developmental factor often used to determine if neonatal intensive care should be offered is whether a 2.5 mm endotracheal tube (ETT) can be successfully placed. There is a belief held among some neonatologists that infants small enough at birth to require the use of a 2.0 mm endotracheal tube (ETT) for resuscitation are too developmentally immature to survive and thus 2.0 mm ETT should not be used because the internal diameter is too small with too high of resistance to flow leading to an inability to provide adequate mechanical ventilation. However, that has not been the experience at the University of Iowa where 2.0 mm ETT are frequently used to care for infants born at 22–23 weeks gestation where the birth weight of these infants is often <550 grams.
However, there is limited information in the literature either supporting or refuting the use of 2.0 mm ETT to provide gas exchange effective enough to support premature infants. A study by Laing regarding prevention of subglottic stenosis in neonatal ventilation used the following infant weight based ETT size recommendations: 2.0 mm ETT for all infants weighing <1.5 kg, 2.5 mm ETT for infants weighing 1.5–2.5 kg, and 3.0 mm ETT for infants weighing 2.5–3.0 kg [9]. Although these endotracheal tube size recommendations were not absolute, the authors reported achieving adequate ventilation in the majority of the 500 infants intubated in their study based on these recommendations. In addition, they reported that the majority of very low birth-weight infants were managed exclusively with 2.0 mm ETT [9]. However, American Academy of Pediatrics neonatal resuscitation guidelines (NRP) do not even mention 2.0 mm ETT, recommending the use of 2.5 mm ETT for infants born at less than 28 weeks gestation and below 1000 g [10].
Given the lack of information in the literature regarding the use of 2.0 mm ETT in extremely premature and extremely low birth-weight infants, in addition to the discrepancy between conventional thinking in the field of neonatology and our institutional practices, we sought to delineate the survival and respiratory outcomes of infants initially intubated with a 2.0 mm ETT compared to a 2.5 mm ETT. The present study was designed to compare the survival and short-term respiratory outcomes of infants weighing <750 g at birth initially intubated with 2.0 mm vs 2.5 mm ETT. The hypothesis underlying this work is that infants initially intubated with a 2.0 mm ETT can be successfully cared for on a ventilator and despite being more premature and smaller will have similar survival and short-term respiratory outcomes as those infants initially intubated with a 2.5 mm ETT.
Methods
After obtaining Institutional Review Board approval, a retrospective cohort analysis was performed of infants born at the University of Iowa between July 1, 2012 and June 30, 2017 with a birth weight <750 g. Infants were grouped by the size of the endotracheal tube (ETT) placed at the first intubation. Infants were excluded if they were born outside the study institution or parents declined active resuscitation. No infants were excluded from the study because of recognized syndromes or congenital malformations. We chose 750 g as our cutoff as no infants larger than that were intubated with a 2.0 ETT during this time.
Infants were routinely resuscitated if estimated gestational age was at least 24 weeks, with parental option to decline resuscitation at 22 or 23 weeks, and no active resuscitations attempted at less than 22 weeks estimated gestational age. Prenatal resuscitation counseling was not based on estimated fetal weight. Neonatal resuscitation in the delivery room was performed by teams led by neonatology fellows and/or attending neonatologists. Infants were intubated in the delivery room if required based on standard NRP criteria. Some infants who did not require intubation in the delivery room were later intubated for progressive respiratory failure on Nasal CPAP or for surfactant replacement. Sheridan uncuffed endotracheal tubes and Sheridan Sher-I-Slip 5Fr stylets (Teleflex Morrisville, NC) are used in our institution. The endotracheal tube size and whether a stylet was used for the initial intubation was chosen by the intubating provider, and both 2.0 and 2.5 mm ETT and stylets are available in both the delivery room and the NICU. The Bunnell Life Pulse high frequency jet ventilator (Bunnell Incorporated Salt Lake City, UT) is the standard ventilator used in our center for infants <750 g and requires the use of a 2.5 mm LifePortTM Endotracheal tube adapter, which contains the side port through which high velocity jets of gas are delivered. The company does not manufacture adaptors for 2.0 mm ETT, but we have found that the 2.5 mm adaptor also fits into a 2.0 mm ETT, with the use of gentle dilation of the proximal end of the ETT with a hemostat to aid insertion. All intubated infants have in-line suction catheters placed and we routinely use 5fr (1.6 mm) Halyard Closed Suction System with Y endotracheal tube adapters (O&M Halyard, Inc Alpharetta, GA) with both 2.0 mm and 2.5 mm ETT tubes. All infants in this study were treated with early surfactant replacement therapy with Survanta® (beractant) (4 mL/kg, divided in 4 equal aliquots) per the institution’s respiratory management protocol. Surfactant is administrated in a two-person procedure with the bedside nurse instilling the surfactant through a 5Fr Halyard Multi-Access Catheter (O&M Halyard, Inc Alpharetta, GA) while the respiratory therapist provides positive pressure ventilation using a neonatal anesthesia bags with similar FiO2, PIP, and PEEP as set on the jet ventilator. The method of surfactant administration does not change based on ETT size and infants usually tolerate this procedure very well.
Neonatal characteristics, treatment, and diagnoses were abstracted from the University of Iowa NICU registry and the electronic health record and collected in a RedCap database. Gestational age was determined by best obstetric estimate using last menstrual period, prenatal ultrasonography, or physical examination. Birth weight was measured using the electronic scales on the infant beds on admission to the NICU. Antenatal steroid therapy was defined as any corticosteroid administered to the pregnant mother prior to delivery for the purpose of enhancing fetal lung maturity.
Neonatal respiratory data were collected, including number of intubation attempts, mode of ventilation and settings, number of inadvertent extubation events or tube malfunctions, days on mechanical ventilation, age in days and postmenstrual age (PMA) at first extubation attempt, age and PMA at final extubation, age at weaning to low flow oxygen, days on continuous positive airway pressure (CPAP), and days on noninvasive ventilation. Days on CPAP included single prong nasopharyngeal CPAP, RAM cannula, and high flow nasal cannula modalities. Noninvasive ventilation included noninvasive neurally adjusted ventilatory assist (NIV-NAVA) or nasal pharyngeal intermittent mandatory ventilation (NP-IMV). Respiratory complications were documented including pneumothorax with and without chest tube placement, pulmonary interstitial emphysema, pulmonary hemorrhage, pneumonia, bronchopulmonary dysplasia, airway anomaly/stenosis, or tracheostomy.
Bronchopulmonary dysplasia (BPD) was defined using the classification system proposed by Jensen et al based on respiratory support in use at 36 weeks PMA [11]. Grade 1 BPD is defined as nasal cannula O2 at ≤2 L per minute flow, Grade 2 as high flow nasal cannula, CPAP, or noninvasive ventilation, and Grade 3 as invasive mechanical ventilation via endotracheal tube. Airway anomalies were included only if surgical intervention was required. The primary outcome of this study was survival to hospital discharge or transfer. The secondary outcomes were short-term respiratory outcomes and complications.
Statistical analysis
Demographic, treatment, survival, and respiratory outcomes were compared between the infants initially intubated with a 2.0 mm ETT and 2.5 mm ETT using Wilcoxon rank-sum tests for continuous variables and Chi-squared or Fisher’s exact test for categorical variables. Ordinal logistic regression and generalized linear models were used to perform multivariable analyses of categorical and continuous outcomes as appropriate. Differences were considered significant when the p-value was less than 0.05. All analyses were performed using SAS software, version 9.4.
Results
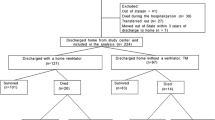
A total of 224 infants were born at the University of Iowa from July 1, 2012 through June 30, 2017 with a birth weight of <750 g. There were 59 infants who were stillborn or experienced intrauterine fetal demise, and 16 who died in the delivery room after comfort care (15 infants were born at less than 22 weeks gestation and active resuscitation was not offered, and 1 infant was born at 22 weeks gestation with multiple anomalies that parents declined resuscitation). There were 149 infants who received active resuscitation and were admitted to the NICU. Of these 149 infants, 69 (46%) were initially intubated with a 2.0 mm ETT and 78 (53%) were intubated with a 2.5 mm ETT. There were two infants that never required endotracheal intubation. A total of 147 infants were included in the final analysis.
A comparison of demographic factors is presented in Table 1. Infants who were intubated with a 2.0 mm ETT were born at significantly lower gestational ages (median 23 weeks (22, 24) vs. 24 weeks (24, 25) p < 0.0001) and had significantly lower birth weights (median 545 g (450, 616) vs 648 g (579, 700) p < 0.001). There was no significant difference in the sex or race between the two groups. The number of infants in each group that survived to discharge was not significantly different (p = 0.0916) even without adjusting for the significant differences in gestational age and birth weight, with 77% (53/69) of the infants intubated with a 2.0 mm ETT surviving to discharge compared to 87% (68/78) of the infants intubated with a 2.5mmETT.
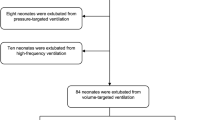
Respiratory variables are presented in Table 2. The infants intubated with a 2.0 mm ETT were more likely to be intubated in the delivery room (96% vs 68%, p < 0.0001) and had lower 1 min and 5 min APGAR scores (3 (2, 4) and 7 (5, 7) for 2.0 mm ETT group vs. 4 (3, 6) and 7 (6, 8) for 2.5 mm ETT group, p < 0.0001 and p = 0.0055, respectively). The number of intubation attempts needed for first successful intubation were similar between groups, with 72% of 2.0 mm ETT and 82% of 2.5 mm ETT infants intubated on first or second attempts, and only 7% of 2.0 mm and 5% of 2.5 mm infants requiring more than three attempts. Almost all infants (97%), irrespective of endotracheal tube size, were placed on first intention high frequency jet ventilation for their initial mode of ventilation (100% vs 94%), consistent with the study institution’s initial ventilator management protocol. Despite having similar depth of intubation in both groups (mean of 6.5 cm (6, 6.5) for both groups p = 0.1511), infants in the 2.0 mm ETT group were more likely to experience at least one unintended extubation (51% vs 34% p = 0.0333). The infants in the 2.0 mm ETT group were also more likely to have their endotracheal tubes electively changed to a larger sized endotracheal tube (55% vs 12%, p < 0.0001) but it occurred at a similar age in both groups (27 vs 24 days of age, p = 0.8392). There was a similar rate of endotracheal tube malfunction in both groups (19% vs 16%, p = 0.6174). Infants in the 2.0 mm ETT group were older in days, at the first trial of extubation (52 (43, 61) vs 43 (26, 52) p = 0.0014), older in days at final extubation (69 (51, 85) vs 50 (39, 72) p = 0.0027), older in days when weaned to low flow oxygen (116 (98, 133) vs 96 (75, 120) p = 0.0035), and required more total ventilator days (61 (39, 74) vs 45 (30, 65). However, when adjusted for gestational age at birth, there were no significant differences in age at 1st extubation (p = 0.2750), either age (p = 0.3977) or PMA (p = 0.4828) at final extubation; and total ventilator days (p = 0.7338).
Ventilator settings are compared between groups in Table 3. In the first 6–12 h of life, infants in the 2.0 mm ETT group required higher Jet PIP (5 cm H20 difference, p < 0.0001), and higher Jet rate (p = 0.01) than those in the 2.5 mm ETT group, but MAP and FiO2 were similar. At 7 days of age, most infants were still being treated with Jet ventilation (n = 117), and infants in the 2.0 mm ETT group were still requiring higher Jet PIP (30 cm H20 vs 26 cm, p = 0.0012) than those in the 2.5 mm ETT group, and also required slightly higher FiO2 (0.35 vs 0.32, p = 0.0024), and MAP (8 cm H20 vs 7 cm, p = 0.0348). Blood gas pCO2 was similar between groups at both 6–12 h and 7 days of age.
Respiratory complications are shown in Table 4. There was no significant difference in the rates of pneumothorax, pulmonary interstitial emphysema, pulmonary hemorrhage, pneumonia, or need for tracheostomy between the two groups. BPD was ubiquitous, with all survivors requiring supplemental O2 at 36 weeks, regardless of ETT group. Many infants in both groups had grade 2 BPD (nasal cannula > 2 LPM, nasal CPAP or noninvasive ventilation) at 36 weeks (77% of 2.0 mm ETT group, 54% of 2.5 mm ETT group). Unadjusted analyses for BPD grade demonstrated worse BPD with 2.0 mm ETT usage (p = 0.0196), which was expected based on the significantly lower GA; however, upon adjustment for gestational age at birth, BPD grade was similar between groups (p = 0.2549). Incidence of grade 3 BPD was both low and equivalent in both ETT groups (12% vs. 13%, p = 1.0).
Discussion
In our 5-year cohort study of inborn infants with birth weight <750 g, survival was similar between infants initially intubated with a 2.0 mm ETT and those intubated with a 2.5 mm ETT despite the infants with a 2.0 mm ETT being significantly smaller and more premature. Survival rates in both high-risk groups were high, 77% of infants intubated with a 2.0 mm ETT and 87% of infants intubated with a 2.5 mm ETT survived to discharge. Although it is a commonly held belief that infants who are small enough to require intubation with a 2.0 mm ETT are not viable, our data demonstrate that the survival rate in this group is similar to the survival rate of infants intubated with a larger, 2.5 mm ETT. The combined survival rate for both groups during this period was >77%. When categorized by gestational age at birth the survival rates for infants in this study were: 65% at 22 weeks, 85% at 23 weeks, 88% at 24 weeks, 89% at 25 weeks, and 89% at 26–28 weeks. This rate of survival is markedly higher than the rate of survival reported by the NICHD Neonatal Research Network (NRN) centers, which in 2010 reported survival rates of 6% for infants born at 22 weeks gestation, 26% for infants born at 23 weeks gestation, 55% for infants born at 24 weeks gestation, and 72% for infants born at 25 weeks gestation [12]. The high rate of survival in our population was likely due to a strong commitment to proactive prenatal and neonatal care provided at the University of Iowa as previously described by Kyser et al. [13]. In our study, 97% of infants received at least one dose of antenatal steroids which stands in contrast to the NICHD NRN centers, that reported antenatal steroid administration to only 13% of 22 week infants, 53% of 23 week infants, and 85% of 25 week infants [12]. While there are currently no official guidelines, recent studies have suggested that antenatal corticosteroids have a beneficial effect when administered at less than 24 weeks of gestation [14,15,16].
One of the main objections to using 2.0 mm ETT size is the belief that adequate oxygenation and ventilation cannot be provided through these devices. However, this study demonstrated that using high frequency jet ventilation, adequate ventilation, and oxygenation can be provided through a 2.0 mm ETT, with the only significant difference being that infants intubated with a 2.0 mm ETT, required as expected, due to the increased resistance to flow, higher peak inspiratory pressures (PIP) and ventilator rates to maintain the same level of alveolar ventilation with no difference in the carbon dioxide levels achieved and minimal difference in the fraction of inspired oxygen required. The need for higher peak pressures and rates is not only due to the increased resistance of the 2.0 mm ETT compared to the 2.5 mm ETT, but to the developmentally more immature lungs in the 2.0 mm ETT group with a median GA of 23 weeks. Since patients with 2.0 mm ETT in this study were treated with high frequency jet ventilation, we cannot comment on the efficacy when using a 2.0 mm ETT with other modes of ventilation including conventional ventilation.
Another argument commonly made for not using 2.0 mm ETT is that they are associated with high rates of plugging and malfunction. However, in our study, we did not find any increased risk of endotracheal tube malfunction or plugging using 2.0 mm ETT compared to 2.5 mm ETT. Sheridan uncuffed endotracheal tubes are the standard ETT used in our NICU which are left full length and uncut, but the specific brand and type of uncuffed endotracheal tube likely does not matter. If the intubating providing prefers to use a stylet when intubating, the Sheridan Sher-I-Slip 5Fr stylets are used in our unit and they easily fit into 2.0 mm ETTs but careful attention is needed to ensure the tip of the stylet is placed 1 cm proximal to the tip of the ETT and does not extend beyond the end of the ETT. We use 5fr Halyard Closed Suction System with Y tube adapters for in-line suction with both 2.0 and 2.5 mm ETT tubes for airway clearance and to ensure patency of the tube, because the external diameter of this suction catheter is 1.66 mm, which allows it to easily pass through 2.0 mm ETT. Although kinking of ETT has been reported, especially with 2.0 mm ETT, we did not have issues with ETTs kinking in our study. All sizes of ETT have the possibility of kinking with the jet ventilator because the end of the ETT where the life port adaptor attaches is a weak spot. However, this can be prevented with good positioning of the jet circuit. Additionally, surfactant administration is always performed at our institution while infants receive manual positive pressure ventilation via a neonatal flow-inflating (anesthesia) bag device with a peep valve, thus we are unable to comment on tube patency during surfactant administration using other ventilatory methods, such as allowing the ventilator to distribute the surfactant, or through the use of a Neo-Puff or other t-piece device.
Previous work by Brown found higher absolute event rates of unintended extubation in infants weighing <1500 g when compared to those weighing >1500 g, although when adjusted for days of intubation there was no difference [17]. In our population, we found infants initially intubated with a 2.0 mm ETT had higher rates of unintended extubation at 0.95 per 100 intubation days compared to 0.86 in the 2.5 mm ETT group, which compares favorably to reports in the literature ranging from 0.14–5.3 per 100 intubations days [18]. Poor fixation of the endotracheal tube has been reported to be the most common cause of unintended extubation [19]. At our institution, endotracheal tubes are secured with two pieces of elastic tape, one longer than the other, each split into an H and sprayed with adhesive spray, with one side taped to the middle-upper lip and the other side taped to the tube. The shorter piece of tape is placed directly on the skin above the upper lip and the longer piece is secured on top. Chest X-rays are obtained immediately after intubation, following repositioning of endotracheal tubes, with any significant change in respiratory status, and at least daily for the first several days to ensure proper position of the endotracheal tube. Tape is changed when it is soiled or no longer adhesive, and this is done as a 2-person procedure by bedside nurses and respiratory therapists.
Infants initially intubated with a 2.0 mm ETT had their ETT electively exchanged for a larger endotracheal tube, usually a 2.5 mm ETT, significantly more often than those initially intubated with a 2.5 mm ETT. Endotracheal tubes were electively exchanged for larger tubes when a sufficiently large air leak developed around the endotracheal tube that hindered the ability to provide optimal oxygenation and ventilation. More than half of the first endotracheal tube changes were planned and elective in nature. However, about 40% of the endotracheal tube changes occurred following an unintended extubation, where a larger tube was used on reintubation. Although there was a trend towards higher ventilator days in infants initially intubated with a 2.0 mm ETT than 2.5 mm ETT (61 vs 45, p = 0.0538), when adjusted for gestational age the difference was no longer significant (p = 0.7338). There was no difference in the days on CPAP, days on noninvasive ventilation, number of infants that needed a tracheostomy, or infants with airway stenosis between the groups.
There were eight infants who were successfully intubated with a 2.0 mm ETT after the initial intubation attempt with a 2.5 mm ETT could not be passed. Six of the eight infants survived to discharge. Had these six infants been born at a center that did not have access to 2.0 mm ETTs the possibility exists that care would have been redirected to comfort and neonatal intensive care would not have been offered. This raises the importance of variation in clinical practices among different institutions and its impact on outcomes. Several studies have demonstrated the level of commitment to proactive management of premature infants at the hospital level could have a significant impact on outcomes [7, 8, 13, 20,21,22,23]. A study by Rysavy et al. of outcomes of infants cared for in the NICHD NRN found that differences in hospital rates of initiating potentially lifesaving treatment after birth in infants born at 22–23 weeks of gestation accounted for 78% of the between-hospital variation in survival and outcomes but only 22% for those born at 24 weeks [22] so management approaches play a role in survival. At our institution, all expecting parents of preterm infants have a prenatal neonatology consult with a neonatology fellow or attending neonatologist to counsel the family on rates of survival and common morbidities associated with prematurity based on the gestational age of infants born at the University of Iowa. For extremely premature infants, the options for resuscitation are discussed with the parents including forgoing active resuscitation of an infant born at 22–23 weeks gestation, although this is a rare choice for families at our institution. During the 5-year study period, there were no infants born between 22–24 weeks gestation without congenital anomalies for whom parents declined active resuscitation. We speculate that this is due to the University of Iowa’s history of providing proactive prenatal and neonatal care along with high rates of survival of extremely premature infants and that families choose to receive medical care here because they desire active resuscitation and intensive neonatal care for their infants.
All surviving infants in our cohort were diagnosed with BPD at 36 weeks, which was not unexpected given their extremely low gestational age at birth. Unadjusted analyses showed that infants in the 2.0 mm ETT group had higher incidence of grade 2 BPD and lower incidence of grade 1 BPD than infants in the 2.5 mm ETT group, but importantly the incidence of grade 3 BPD was the same in both groups. After adjustment for gestational age at birth, there was no significant association between ETT size and BPD classification supporting the concept that the degree of ventilator support provided by the 2.0 mm ETT compared to the 2.5 mm ETT does not significantly impact the development of chronic lung disease in a negative fashion. This is important, as grade 3 BPD, the most severe grade, is strongly associated with early childhood respiratory morbidity and neurodevelopmental impairment [11].
This study has several strengths. To the best of our knowledge, this is the first study to compare the survival and respiratory outcomes of extremely premature and low birth-weight infants initially intubated with a 2.0 mm ETT to those with a 2.5 mm ETT. The second is that this was a single center study, which ensures that all infants during the study period were treated similarly based on a single institution’s practices and policies. Finally, the study shows that premature infants weighing <750 g can be ventilated successfully with the initial use of a 2.0 mm ETT when using high frequency jet ventilation.
We acknowledge that our study has several limitations that should be considered when interpreting the results. The first is that this is a retrospective study using data from our neonatal registry. The second is that the size of the endotracheal tube initially inserted was at the discretion of the practitioner managing the airway as part of neonatal resuscitation and not prospectively randomized; however, the usual approach for infants born at 22 weeks gestation is to intubate using a 2.0 mm ETT. Additionally, there were eight patients who failed initial placement of a 2.5 mm ETT that were successfully resuscitated due to access to a 2.0 mm ETT. The third limitation is that this study was performed at a single level IV academic NICU with a high rate of perinatal steroid administration as early as 22 weeks, majority of families desiring active resuscitation, neonatal survival rates above the national average for extremely premature infants, and almost exclusive use of high frequency jet ventilation in premature infants. Thus, our results may not be generalizable to other institutions using other modes of ventilation. Lastly, this study was limited to the survival and short-term respiratory outcomes and therefore further studies need to be done to assess the long-term respiratory and neurodevelopmental outcomes of infants initially intubated with a 2.0 mm ETT.
In summary, our analysis found that infants initially intubated with a 2.0 mm ETT had similar survival and short-term respiratory outcomes as those intubated with a 2.5 mm ETT despite being smaller and more premature and importantly could be successfully ventilated with HFJV. In light of recent studies reporting improved survival of infants born at less than 24 weeks of gestation without worsening neurodevelopmental outcomes [24], the use of 2.0 mm ETT should be considered as an initial approach to neonatal resuscitation for infants born at <23 weeks gestation and access to 2.0 mm ETT should be available to all teams that offer resuscitation to infants born at <24 weeks gestation.
References
Ecker JL, Kaimal A, Mercer BM, Blackwell SC, deRegnier RAO, Farrell RM, et al. Periviable birth: interim update. Am J Obstet Gynecol. 2016;215:B2–B12.e1.
Lau C, Ambalavanan N, Chakraborty H, Wingate MS, Carlo WA. Extremely low birth weight and infant mortality rates in the United States. Pediatrics. 2013;131:855–60.
Stoll BJ, Hansen NI, Bell EF, Walsh MC, Carlo WA, Shankaran S, et al. Trends in care practices, morbidity, and mortality of extremely preterm neonates, 1993-2012. JAMA. 2015;314:1039–51.
Younge N, Goldstein RF, Bann CM, Hintz SR, Patel RM, Smith PB, et al. Survival and neurodevelopmental outcomes among periviable infants. N Engl J Med. 2017;376:617–28.
Fayoux P, Devisme L, Merrot O, Marciniak B. Determination of endotracheal tube size in a perinatal population: an anatomical and experimental study. Anesthesiology. 2006;104:954–60.
Bader D, Kugelman A, Boyko V, Levitzki O, Lerner-Geva L, Riskin A, et al. Risk factors and estimation tool for death among extremely premature infants: a national study. Pediatrics. 2010;125:696–703.
Litmanovitz I, Reichman B, Arnon S, Boyko V, Lerner-Geva L, Bauer-Rusak S, et al. Perinatal factors associated with active intensive treatment at the border of viability: a population-based study. J Perinatol. 2015;35:705–11.
Tyson JE, Younes N, Verter J, Wright LL. Viability, morbidity, and resource use among newborns of 501- to 800-g birth weight. National Institute of Child Health and Human Development Neonatal Research Network. JAMA. 1996;276:1645–51.
Laing IA, Cowan DL, Ballantine GM, Hume R. Prevention of subglottic stenosis in neonatal ventilation. Int J Pediatr Otorhinolaryngol. 1986;11:61–6.
In: Weiner GM, Zaichkin J, editors. American Academy of Pediatrics and American Heart Association. Textbook of Neonatal Resuscitation (NRP), 7th edn. American Academy of Pediatrics; 2016:326 p.
Jensen EA, Dysart K, Gantz MG, McDonald S, Bamat NA, Keszler M, et al. The diagnosis of bronchopulmonary dysplasia in very preterm infants. an evidence-based approach. Am J Respir Crit Care Med. 2019;200:751–9.
Stoll BJ, Hansen NI, Bell EF, Shankaran S, Laptook AR, Walsh MC, et al. Neonatal outcomes of extremely preterm infants from the NICHD Neonatal Research Network. Pediatrics. 2010;126:443–56.
Kyser KL, Morriss FH Jr., Bell EF, Klein JM, Dagle JM. Improving survival of extremely preterm infants born between 22 and 25 weeks of gestation. Obstet Gynecol. 2012;119:795–800.
Travers CP, Carlo WA, McDonald SA, Das A, Bell EF, Ambalavanan N, et al. Mortality and pulmonary outcomes of extremely preterm infants exposed to antenatal corticosteroids. Am J Obstet Gynecol. 2018;218:130.e1–e13.
Mori R, Kusuda S, Fujimura M. Antenatal corticosteroids promote survival of extremely preterm infants born at 22 to 23 weeks of gestation. J Pediatr. 2011;159:110–4. e1
Ehret DEY, Edwards EM, Greenberg LT, Bernstein IM, Buzas JS, Soll RF, et al. Association of antenatal steroid exposure with survival among infants receiving postnatal life support at 22 to 25 weeks’ gestation. JAMA Netw Open. 2018;1:e183235.
Brown MS. Prevention of accidental extubation in newborns. Am J Dis Child. 1988;142:1240–3.
Silva PS, Reis ME, Aguiar VE, Fonseca MC. Unplanned extubation in the neonatal ICU: a systematic review, critical appraisal, and evidence-based recommendations. Respir Care. 2013;58:1237–45.
Veldman A, Trautschold T, Weiss K, Fischer D, Bauer K. Characteristics and outcome of unplanned extubation in ventilated preterm and term newborns on a neonatal intensive care unit. Paediatr Anaesth. 2006;16:968–73.
Fanaroff AA, Stoll BJ, Wright LL, Carlo WA, Ehrenkranz RA, Stark AR, et al. Trends in neonatal morbidity and mortality for very low birthweight infants. Am J Obstet Gynecol. 2007;196:147.e1–8.
Hakansson S, Farooqi A, Holmgren PA, Serenius F, Hogberg U. Proactive management promotes outcome in extremely preterm infants: a population-based comparison of two perinatal management strategies. Pediatrics. 2004;114:58–64.
Rysavy MA, Li L, Bell EF, Das A, Hintz SR, Stoll BJ, et al. Between-hospital variation in treatment and outcomes in extremely preterm infants. N Engl J Med. 2015;372:1801–11.
Smith PB, Ambalavanan N, Li L, Cotten CM, Laughon M, Walsh MC, et al. Approach to infants born at 22 to 24 weeks’ gestation: relationship to outcomes of more-mature infants. Pediatrics. 2012;129:e1508–16.
Watkins PL, Dagle JM, Bell EF, Colaizy TT. Outcomes at 18 to 22 months of corrected age for infants born at 22 to 25 weeks of gestation in a center practicing active management. J Pediatr. 2020;217:52–8.
Funding
This work was supported by the University of Iowa. No funding for this research was received.
Author information
Authors and Affiliations
Contributions
All authors participated in the design, editing, and writing of the study. All authors approved the final manuscript. JNB, JMD, and TTC designed the study. JNB extracted the data, interpreted the results, and wrote the manuscript. TGE extracted the data and edited the manuscript. JMD and JMK assisted with content and revision. TTC conducted the statistical analysis of the data, interpreted the results, and created the figures.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Berger, J.N., Elgin, T.G., Dagle, J.M. et al. Survival and short-term respiratory outcomes of <750 g infants initially intubated with 2.0 mm vs. 2.5 mm endotracheal tubes. J Perinatol 42, 202–208 (2022). https://doi.org/10.1038/s41372-021-01227-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41372-021-01227-y
This article is cited by
-
Use of 2.0-mm endotracheal tubes for periviable infants
Journal of Perinatology (2022)