Abstract
Objective
To develop risk prediction models for singleton preterm birth (PTB) < 28 weeks and <32 weeks.
Methods
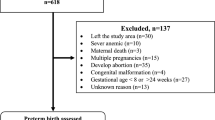
Using a retrospective cohort of 267,226 singleton births in Ontario hospitals, we included variables from the first and second trimester in multivariable logistic regression models to predict overall and spontaneous PTB < 28 weeks and <32 weeks.
Results
During the first trimester, the area under the curve (AUC) for prediction of PTB < 28 weeks for nulliparous and multiparous women was 68.5% (95% CI: 63.5–73.6%) and 73.4% (68.6–78.2%), respectively, while for PTB < 32 weeks it was 68.9% (65.5–72.3%) and 75.5% (72.3–78.7%), respectively. AUCs for second-trimester models were 72.4% (95% CI: 69.7–75.1%) and 78.2% (95% CI: 75.8–80.5%), respectively, in nulliparous and multiparous women. Predicted probabilities were well-calibrated within a wide range around expected base prevalence for the study outcomes.
Conclusions
Our prediction models generated acceptable AUCs for PTB < 28 weeks and <32 weeks with good calibration during the first and second trimester.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Saigal S, Doyle LW. An overview of mortality and sequelae of preterm birth from infancy to adulthood. Lancet Lond Engl. 2008;371:261–9.
Petrou S, Abangma G, Johnson S, Wolke D, Marlow N. Costs and health utilities associated with extremely preterm birth: evidence from the EPICure study. Value Health J Int Soc Pharmacoeconomics Outcomes Res. 2009;12:1124–34.
Henderson J, Carson C, Redshaw M. Impact of preterm birth on maternal well-being and women’s perceptions of their baby: a population-based survey. BMJ Open. 2016;6:e012676.
Behrman RE, Butler AS, Outcomes I of M (US) C on UPB and AH. Societal costs of preterm birth. National Academies Press (US). 2007. https://www.ncbi.nlm.nih.gov/books/NBK11358/. Accessed 29 Jan 2020.
Mangham LJ, Petrou S, Doyle LW, Draper ES, Marlow N. The cost of preterm birth throughout childhood in England and Wales. Pediatrics. 2009;123:e312–327.
Johnson S, Marlow N. Early and long-term outcome of infants born extremely preterm. Arch Dis Child. 2017;102:97–102.
Glass HC, Costarino AT, Stayer SA, Brett C, Cladis F, Davis PJ. Outcomes for extremely premature infants. Anesth Analg. 2015;120:1337–51.
Khan KA, Petrou S, Dritsaki M, Johnson SJ, Manktelow B, Draper ES, et al. Economic costs associated with moderate and late preterm birth: a prospective population-based study. BJOG Int J Obstet Gynaecol. 2015;122:1495–505.
Costeloe KL, Hennessy EM, Haider S, Stacey F, Marlow N, Draper ES. Short term outcomes after extreme preterm birth in England: comparison of two birth cohorts in 1995 and 2006 (the EPICure studies). BMJ. 2012;345. https://www.bmj.com/content/345/bmj.e7976. Accessed 24 Apr 2020.
Sutton L, Bajuk B. Population-based study of infants born at less than 28 weeks’ gestation in New South Wales, Australia, in 1992-3. New South Wales Neonatal Intensive Care Unit Study Group. Paediatr Perinat Epidemiol. 1999;13:288–301.
Sasaki Y, Ishikawa K, Yokoi A, Ikeda T, Sengoku K, Kusuda S, et al. Short- and long-term outcomes of extremely preterm infants in japan according to outborn/inborn birth status. Pediatr Crit Care Med Soc Crit Care Med. 2019;20:963–9.
Woolery LK, Grzymala-Busse J. Machine learning for an expert system to predict preterm birth risk. J Am Med Inf Assoc. 1994;1:439–46.
Goodwin LK, Iannacchione MA, Hammond WE, Crockett P, Maher S, Schlitz K. Data mining methods find demographic predictors of preterm birth. Nurs Res. 2001;50:340–5.
Grobman WA, Lai Y, Iams JD, Reddy UM, Mercer BM, Saade G, et al. Prediction of spontaneous preterm birth among nulliparous women with a short cervix. J Ultrasound Med. 2016;35:1293–7.
Gao C, Osmundson S, Velez Edwards DR, Jackson GP, Malin BA, Chen Y. Deep learning predicts extreme preterm birth from electronic health records. J Biomed Inform. 2019;100:103334.
Weber A, Darmstadt GL, Gruber S, Foeller ME, Carmichael SL, Stevenson DK, et al. Application of machine-learning to predict early spontaneous preterm birth among nulliparous non-Hispanic black and white women. Ann Epidemiol. 2018;28:783–789.e1.
Lemyre B, Moore G. Counselling and management for anticipated extremely preterm birth. Paediatr Child Health. 2017;22:334–41.
Kyser KL, Morriss FH, Bell EF, Klein JM, Dagle JM. Improving survival of extremely preterm infants born between 22 and 25 weeks of gestation. Obstet Gynecol. 2012;119:795–800.
Lee KA, Chang MH, Park M-H, Park H, Ha EH, Park EA, et al. A model for prediction of spontaneous preterm birth in asymptomatic women. J Women’s Health. 2011;20:1825–31.
Lucaroni F, Morciano L, Rizzo G, D’ Antonio F, Buonuomo E, Palombi L, et al. Biomarkers for predicting spontaneous preterm birth: an umbrella systematic review. J Matern-Fetal Neonatal Med. 2018;31:726–34.
Hill JL, Campbell MK, Zou GY, Challis JRG, Reid G, Chisaka H, et al. Prediction of preterm birth in symptomatic women using decision tree modeling for biomarkers. Am J Obstet Gynecol. 2008;198:468.e1–468.e9.
Oskovi Kaplan ZA, Ozgu-Erdinc AS. Prediction of preterm birth: maternal characteristics, ultrasound markers, and biomarkers: an updated overview. J Pregnancy. 2018. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6199875/.
Nikolova T, Uotila J, Nikolova N, Bolotskikh VM, Borisova VY, Di, et al. Prediction of spontaneous preterm delivery in women presenting with premature labor: a comparison of placenta alpha microglobulin-1, phosphorylated insulin-like growth factor binding protein-1, and cervical length. Am J Obstet Gynecol. 2018;219:610.e1–610.e9.
Daskalakis GJ, Papantoniou NE, Koutsodimas NB, Papapanagiotou A, Antsaklis AJ. Fetal fibronectin as a predictor of preterm birth. J Obstet Gynaecol. 2000;20:347–53.
Deshpande SN, van Asselt ADI, Tomini F, Armstrong N, Allen A, Noake C, et al. Rapid fetal fibronectin testing to predict preterm birth in women with symptoms of premature labour: a systematic review and cost analysis. Health Technol Assess Winch Engl. 2013;17:1–138.
Honest H, Bachmann LM, Gupta JK, Kleijnen J, Khan KS. Accuracy of cervicovaginal fetal fibronectin test in predicting risk of spontaneous preterm birth: systematic review. BMJ. 2002;325:301.
Conde-Agudelo A, Romero R. Cervical phosphorylated insulin-like growth factor binding protein-1 test for the prediction of preterm birth: a systematic review and meta-analysis. Am J Obstet Gynecol. 2016;214:57–73.
Preterm birth. 2019. https://www.who.int/news-room/fact-sheets/detail/preterm-birth. Accessed 25 Nov 2019.
Health Statistics Division. Low Birth Weight Newborns in Canada 2000 to 2013: Health Fact Sheets. 2016. https://www150.statcan.gc.ca/n1/pub/82-625-x/2016001/article/14674-eng.htm. Accessed 24 Oct 2019.
Access the BORN Information System. 2020. https://www.bornontario.ca/en/data/access-the-born-information-system.aspx. Accessed 21 Jul 2020.
Miao Q, Fell DB, Dunn S, Sprague AE. Agreement assessment of key maternal and newborn data elements between birth registry and Clinical Administrative Hospital Databases in Ontario, Canada. Arch Gynecol Obstet. 2019;300:135–43.
Ferrero DM, Larson J, Jacobsson B, Di Renzo GC, Norman JE, Martin JN, et al. Cross-country individual participant analysis of 4.1 million singleton births in 5 countries with very high human development index confirms known associations but provides no biologic explanation for 2/3 of all preterm births. PLoS ONE. 2016;11:e0162506.
Frey HA, Klebanoff MA. The epidemiology, etiology, and costs of preterm birth. Semin Fetal Neonatal Med. 2016;21:68–73.
Plagge T, McKinney D, Defranco EA, Adcock R, Kelly E. 486: Contribution of risk factors to extreme preterm birth. Am J Obstet Gynecol. 2019;220:S325–6.
Institute of Medicine (US) Committee on Understanding Premature Birth and Assuring Healthy Outcomes. Preterm Birth: Causes, Consequences, and Prevention. In: Behrman RE, Butler AS, editors. Washington (DC): National Academies Press (US); 2007. http://www.ncbi.nlm.nih.gov/books/NBK11362/ (The National Academies Collection: Reports funded by National Institutes of Health). Accessed 1 Apr 2020.
Gilani N, Haghshenas R, Esmaeili M. Application of multivariate longitudinal models in SIRT6, FBS, and BMI analysis of the elderly. Aging Male. 2019;22:260–5.
Pillay P, Moodley K, Moodley J, Mackraj I. Placenta-derived exosomes: potential biomarkers of preeclampsia. Int J Nanomed. 2017;12:8009–23.
Jelliffe-Pawlowski LL, Shaw GM, Currier RJ, Stevenson DK, Baer MsRJ, O’Brodovich HM, et al. Association of early preterm birth with abnormal levels of routinely collected first and second trimester biomarkers. Am J Obstet Gynecol. 2013;208:492.e1–492.e11.
Waller DK, Lustig LS, Cunningham GC, Feuchtbaum LB, Hook EB. The association between maternal serum alpha-fetoprotein and preterm birth, small for gestational age infants, preeclampsia, and placental complications. Obstet Gynecol. 1996;88:816–22.
WHO | Obesity: preventing and managing the global epidemic. Geneva; 2000. http://www.who.int/entity/nutrition/publications/obesity/WHO_TRS_894/en/index.html (Report of a World Health Organization Consultation). Report No.: 894. Accessed 23 Oct 2019.
Institute of Medicine (US) and National Research Council (US) Committee to Reexamine IOM Pregnancy Weight Guidelines. Weight Gain During Pregnancy: Reexamining the Guidelines. In: Rasmussen KM, Yaktine AL, editors. Washington (DC): National Academies Press (US); 2009. http://www.ncbi.nlm.nih.gov/books/NBK32813/ (The National Academies Collection: Reports funded by National Institutes of Health). Accessed 1 Oct 2019.
Oliver-Williams C, Fleming M, Wood AM, Smith G. Previous miscarriage and the subsequent risk of preterm birth in Scotland, 1980–2008: a historical cohort study. BJOG Int J Obstet Gynaecol. 2015;122:1525–34.
Cavoretto P, Candiani M, Giorgione V, Inversetti A, Abu-Saba MM, Tiberio F, et al. Risk of spontaneous preterm birth in singleton pregnancies conceived after IVF/ICSI treatment: meta-analysis of cohort studies. Ultrasound Obstet Gynecol. 2018;51:43–53.
Gagnon A, Wilson RD. Society of Obstetricians and Gynaecologists of Canada Genetics Committee. Obstetrical complications associated with abnormal maternal serum markers analytes. J Obstet Gynaecol Can. 2008;30:918–32.
Yaron Y, Cherry M, Kramer RL, O’Brien JE, Hallak M, Johnson MP, et al. Second-trimester maternal serum marker screening: maternal serum alpha-fetoprotein, beta-human chorionic gonadotropin, estriol, and their various combinations as predictors of pregnancy outcome. Am J Obstet Gynecol. 1999;181:968–74.
Odibo AO, Sehdev HM, Stamilio DM, Macones GA. Evaluating the thresholds of abnormal second trimester multiple marker screening tests associated with intra-uterine growth restriction. Am J Perinatol. 2006;23:363–7.
Moons KGM, Altman DG, Reitsma JB, Ioannidis JPA, Macaskill P, Steyerberg EW, et al. Transparent Reporting of a multivariable prediction model for Individual Prognosis or Diagnosis (TRIPOD): explanation and elaboration. Ann Intern Med. 2015;162:W1–73.
Batista GEAPA, Prati RC, Monard MC. A study of the behavior of several methods for balancing machine learning training data. SIGKDD Explor Newsl. 2004;6:20–9.
Fotouhi S, Asadi S, Kattan MW. A comprehensive data level analysis for cancer diagnosis on imbalanced data. J Biomed Inform. 2019;90:103089.
Robin X, Turck N, Hainard A, Tiberti N, Lisacek F, Sanchez J-C, et al. pROC: Display and Analyze ROC Curves. 2019. https://CRAN.R-project.org/package=pROC. Accessed 30 Sep 2019.
Steyerberg EW, Vickers AJ, Cook NR, Gerds T, Gonen M, Obuchowski N, et al. Assessing the performance of prediction models: a framework for some traditional and novel measures. Epidemiol Camb Mass. 2010;21:128–38.
Stuart EA, Azur M, Frangakis C, Leaf P. Multiple imputation with large data sets: a case study of the children’s mental health initiative. Am J Epidemiol. 2009;169:1133–9.
Hosmer, DW, Lemeshow S. Applied logistic regression. Second edition. John Wiley & Sons, Inc.; 2000.
Meertens LJE, Montfort P, van, Scheepers HCJ, Kuijk SMJ, van, Aardenburg R, Langenveld J, et al. Prediction models for the risk of spontaneous preterm birth based on maternal characteristics: a systematic review and independent external validation. Acta Obstet Gynecol Scand. 2018;97:907–20.
Akobeng AK. Understanding diagnostic tests 3: receiver operating characteristic curves. Acta Paediatr. 2007;96:644–7.
Pepe MS, Cai T, Longton G. Combining predictors for classification using the area under the receiver operating characteristic curve. Biometrics. 2006;62:221–9.
Melchor JC, Khalil A, Wing D, Schleussner E, Surbek D. Prediction of preterm delivery in symptomatic women using PAMG-1, fetal fibronectin and phIGFBP-1 tests: systematic review and meta-analysis. Ultrasound Obstet Gynecol. 2018;52:442–51.
Christodoulou E, Ma J, Collins GS, Steyerberg EW, Verbakel JY, Van, et al. A systematic review shows no performance benefit of machine learning over logistic regression for clinical prediction models. J Clin Epidemiol. 2019;110:12–22.
Macdonald-Wallis C, Silverwood RJ, Stavola BL de, Inskip H, Cooper C, Godfrey KM, et al. Antenatal blood pressure for prediction of pre-eclampsia, preterm birth, and small for gestational age babies: development and validation in two general population cohorts. BMJ. 2015;351. https://www.bmj.com/content/351/bmj.h5948. Accessed 23 Mar 2020.
Johnson JM, Khoshgoftaar TM. Survey on deep learning with class imbalance. J Big Data. 2019;6:27.
Gilani N, Kazemnejad A, Zayeri F, Yazdani. Comparison of marginal logistic model with repeated measures and conditional logistic model in risk factors affecting hypertension. J Mazandaran Univ Med Sci. 2011;21:27–35.
Gilani N, Kazemnejad A, Zayeri F, Asghari Jafarabadi M, Izadi Avanji FS. Predicting outcomes in traumatic brain injury using the glasgow coma scale: a joint modeling of longitudinal measurements and time to event. Iran Red Crescent Med J. 2017;19.
Shanab AA, Khoshgoftaar TM, Wald R, Napolitano A. Impact of noise and data sampling on stability of feature ranking techniques for biological datasets. In: 2012 IEEE 13th International Conference on Information Reuse Integration (IRI). 2012. p. 415–22.
Acknowledgements
We greatly appreciate the assistance of our Associate Editor and two anonymous referees for careful reading and valuable suggestions on our manuscript that significantly improved the presentation of the paper. This work was supported by the Canadian Institutes of Health Research (CIHR; grant #: 151520). Dr. McDonald is supported by a Tier II CIHR Canada Research Chair (950-229920). Dr. Beyene holds the John D. Cameron Endowed Chair in the Genetic Determinants of Chronic Diseases, McMaster University. CIHR had no role in the design or conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; or the decision to submit the manuscript for publication.
Funding
This work was supported by the Canadian Institutes of Health Research (CIHR; grant #: 151520). Dr. McDonald is supported by a Tier II CIHR Canada Research Chair (950-229920). Joseph Beyene holds the John D. Cameron Endowed Chair in the Genetic Determinants of Chronic Diseases, McMaster University. CIHR had no role in the design or conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; or the decision to submit the manuscript for publication.
Author information
Authors and Affiliations
Contributions
RAB conducted the literature review and statistical analysis and drafted the manuscript. SDM and JB provided clinical and analytical feedback, and RAB incorporated feedback. All authors approved the final version of the paper before its submission.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Arabi Belaghi, R., Beyene, J. & McDonald, S.D. Clinical risk models for preterm birth less than 28 weeks and less than 32 weeks of gestation using a large retrospective cohort. J Perinatol 41, 2173–2181 (2021). https://doi.org/10.1038/s41372-021-01109-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41372-021-01109-3
This article is cited by
-
Node embedding-based graph autoencoder outlier detection for adverse pregnancy outcomes
Scientific Reports (2023)
-
PredictPTB: an interpretable preterm birth prediction model using attention-based recurrent neural networks
BioData Mining (2022)
-
Prediction of low Apgar score at five minutes following labor induction intervention in vaginal deliveries: machine learning approach for imbalanced data at a tertiary hospital in North Tanzania
BMC Pregnancy and Childbirth (2022)