Abstract
Objective
To examine the extents to which low tidal volume (VT) and endotracheal tube (ETT) leakeage influence the accuracy of ETCO2 for estimating arterial PCO2 (PaCO2) in very low birth weight (VLBW) infants with mechanical ventilation.
Study design
An observational study. We evaluated a total of 287 paired ETCO2 and PaCO2 values as well as VTs obtained from 22 VLBW infants with ventilation. Deming regression, quadratic discriminant analysis, and Bland–Altman analysis were performed.
Result
ETCO2 and PaCO2 were correlated (r2 = 0.5897, p < 0.0001). A quadratic discrimination analysis of the VT and the percentage of leak yielded 70.4% [95%CI, 65.1 to 75.7] discrimination for the agreement between ETCO2 and PaCO2. ETCO2 was strongly correlated with PaCO2 in the discriminant function Z > 0 group (r2 = 0.7234, p < 0.0001).
Conclusion
Our results indicate that ETCO2 is a good surrogate for PaCO2 when VT is high and ETT leak is low.
Similar content being viewed by others
Introduction
The preterm lung is highly fragile because the lung is structurally immature and deficient in surfactant, and lung overdistension can cause pulmonary inflammation leading to lung injury. Therefore, careful monitoring of respiratory mechanics, including CO2, is necessary during mechanical ventilation to prevent volutrauma and chronic lung disease[1, 2].
Capnography, which displays the level and waveform of CO2 in expired breaths, is a simple and well-established standard monitoring technique that indicates arterial PCO2 (PaCO2) and provides information on cell metabolism, blood perfusion, and alveolar ventilation in adult and pediatric patients [3, 4]. The use of end-tidal CO2 (ETCO2) for monitoring and as a tool for verifying endotracheal tube (ETT) position is another standard technique [4,5,6].
Several investigators have demonstrated that ETCO2 was in good correlation with PaCO2 values in infants, including extremely low birth weight infants [7,8,9,10,11,12]. However, capnography has not been widely accepted by neonatologists for physiological and technical reasons, such as the weight of sensors or water droplets within circuits, dead space, and leakage from tracheal intubation tubes [13, 14]. Furthermore, the difficulty in acquiring adequate CO2 waveforms due to tachypnea and ventilation–perfusion mismatch affects the accuracy of ETCO2.
The main problem with capnography in infants is the low tidal volume (VT) and leakage from tracheal intubation, which is often observed when using uncuffed ETTs [15]. While the effects of ETT leakage on the monitoring of VT and respiratory mechanics have been well investigated, little is known about the extents to which low VTs and ETT leakage influence the agreement between ETCO2 and PaCO2. The aim of this study was to evaluate the accuracy of ETCO2 for estimating PaCO2 and examine the effects of different amounts of ETT leakage and inspiratory VTs on ETCO2 measurements in very low birth weight (VLBW) infants requiring mechanical ventilation.
Materials and methods
This is an observational study and the study subjects consisted of 22 VLBW infants admitted to our Neonatal Intensive Care Unit at Fukuda Hospital, Kumamoto, Japan, and treated with mechanical ventilation during the period from March 2013 to February 2015. Data were excluded when waveform capnography was not available. Demographics, clinical features, and laboratory test result data for each subject were collected from medical charts.
The infants were mechanically ventilated with synchronized intermittent mandatory ventilation with pressure support (SIMV + PSV) using a time-cycled pressure-limited ventilator (Puritan BennettTM 840 ventilator®; Medtronic, Mansfield, MA, USA). The peak inspiratory pressure, support pressure, oxygen concentration, inspiratory time, positive end expiratory pressure, and respiratory rate were arranged to obtain the optimal arterial PaO2 and PaCO2 as determined by the physicians.
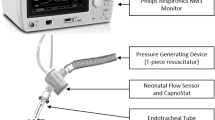
ETCO2 was measured through a mainstream capnometer, which is lightweight (4 g) and has a low dead space (0.5 mL) airway adapter, connected to the proximal end of the endotracheal tube (cap-ONE®, TG-970P; Nihon-Kohden, Tokyo, Japan). Data were continuously recorded on a laptop computer using a software programmed by LabVIEW (National Instruments, Texas, USA) through a CO2 monitor (OLG-2800; Nihon-Kohden, Tokyo, Japan) in each subjects. ETCO2 varied appreciably from breath to breath in the presence of spontaneous breathing during mechanical ventilation. However, as previously described [8], there is a good correlation between PaCO2 and maximum ETCO2 of 20 s capnography. Therefore, the maximum ETCO2 values were chosen based on the results of capnography for 20 s at the same time as the blood gas analysis.
The respiratory monitoring data, such as the VT and the leakage volume, were continuously downloaded from the ventilator by using a software 840 DCI (Medtronic, Mansfield, Massachusetts, USA) with a sampling rate of 6 s. These parameters were measured at the same time as the blood gas analysis. The percentage of ETT leakage was calculated using the following equation:
All the blood samples were drawn from indwelling arterial lines into a 0.1-mL heparinized syringe to prevent coagulation. PaCO2 measurements were then made immediately using a bedside blood gas analytical instruments (ABL 700; Radiometer, Copenhagen, Denmark). All blood gas analyses were performed as part of the subject’s evaluation (including PaO2, PaCO2, electrolytes, or lactate, etc.). Calibrations were performed automatically for the blood gas analyzer, and the accuracy of the capnography was examined using a 5% CO2 gas cylinder. Zero calibration was also performed according to the operator’s manual before the capnography device was connected to the respiration circuit.
Statistical analysis
All the statistical analyses were performed using MedCalc software version 16.8 (MedCalc Software bvba, Ostend, Belgium).
To determine whether ETCO2 was representative of PaCO2, the correlation between ETCO2 and PaCO2 was analyzed by Deming regression [16]. Deming regression is a preferred method for comparing two analytic methods or the same method at different time points. Because it considers both x- and y-axes to be subject to measurement error, it is less influenced by outliers. Bland–Altman plots [17] were conducted to assess the agreement between ETCO2 and PaCO2.
A quadratic discriminant analysis [18] was also used to assess whether the agreement between ETCO2 and PaCO2 was influenced by respiratory monitoring data about the VT and the percentage of ETT leakage. The quadratic discriminant function (Z = 0) is the boundary that separates into two groups; positive values of the discriminant function (Z > 0) are associated with the group that has an agreement between ETCO2 and PaCO2. And if the discriminant function is negative (Z < 0), there is no agreement between ETCO2 and PaCO2. We defined a bias (ETCO2–PaCO2) level of −6.74 mmHg as the acceptable lower limit for the difference between ETCO2 and PaCO2 based on our previous experiments examining ETCO2 in rabbits [7], which showed that the limits of agreement were −6.74 to 4.99 mmHg based on Bland–Altman plots. Values of p < 0.05 were determined to be significant.
Statement of ethics
Informed consent was obtained from the subjects’ guardians, and this study was performed under the control of the Ethics Committee of Medicine and Medical Care, Fukuda Hospital, Kumamoto, Japan.
Results
A total of 287 paired ETCO2 and PaCO2 values obtained from 22 VLBW infants were compared. The median gestational age, birth weight of subjects, and postnatal days at the measurement performed were 27 weeks (range 25–34 weeks), 944 g (range 643–1499 g) and 4 days (range 1–30 days), respectively. The median and range of VT were 8.9 mL (range 2.2 to 27.6 mL), and the percentage of ETT leak was 2.2% (range 0–60.7%).
Figure 1 shows the Deming regression analysis (left) and Bland-Altman plot (right). The correlation between ETCO2 and PaCO2 was statistically significant (p < 0.0001); however, these were not practically relevant (r2 = 0.5897). In the Bland–Altman plot analysis, the mean difference (bias) and the standard deviation (SD) of the differences for ETCO2 were –5.94 ± 6.63 mmHg [95% CI, −18.9 to 7.05]. In particular, ETCO2 can be used to estimate PaCO2 in the group with a TV of 8 mL or more and a ETT leak of 7% or less (n = 156, r2 = 0.7621, p < 0.0001).
The correlation between ETCO2 and PaCO2 (a) was statistically significant (p < 0.0001); however, there is no practically relevant (r2 = 0.5897). In the Bland–Altman plot test (b), the mean difference (bias) and the SD of the differences for ETCO2 was −5.94 ± 6.63 mmHg (95% CI, −18.9 to 7.05 mmHg). The solid line and the dashed lines represent the mean and ±1.96 SD.
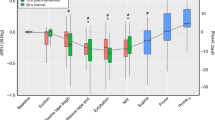
Figure 2 shows the quadratic discriminant analysis for VT and percentage of ETT leak. The differences between ETCO2 and PaCO2 (bias) were compared to the bias of a previous study [7] to determine the degree of agreement between ETCO2 and PaCO2. A discrimination analysis for the VT and the percentage of ETT leak yielded 70.4% [95% CI, 65.1 to 75.7] discrimination for the agreement between ETCO2 and PaCO2. The solid line shows the discriminant function, which decides whether they agreed or not by a quadratic discrimination analysis of the VT and the percentage of the ETT leak. This figure shows that the VT and the percentage of ETT leakage provide independent information for evaluating the agreement between ETCO2 and PaCO2.
The differences between ETCO2 and PaCO2 (bias) were compared to the bias of a previous study [7] to determine the degree of agreement between ETCO2 and PaCO2. The open circles represent measurements where the ETCO2 and PaCO2 measurements were not correlated; the solid circles represent measurements where the ETCO2 measurement was correlated with the PaCO2 measurement. A discrimination analysis for the tidal volume and the percentage of leak yielded 70.4% [95% CI, 65.1–75.7] discrimination for the agreement between ETCO2 and PaCO2. The solid line shows the discriminant function, which decides whether they agreed or not by a quadratic discrimination analysis of the VT and the percentage of the leak. This figure shows that the tidal volume and the percentage of leak provide independent information for evaluating the agreement between ETCO2 and PaCO2. VLBW: very low birth weight, ETT: end tracheal tube.
We also classified the data into two groups, Z < 0 and Z > 0 groups, based on the results of a quadratic discriminant function. ETCO2 was strongly correlated with PaCO2 in the discriminant function Z > 0 group (r2 = 0.7234, p < 0.0001, Fig. 3a). The p value is less than 0.001 in the Z < 0 group as well; however, the correlation between ETCO2 and PaCO2 was weak (r2 = 0.6352) (Fig. 3b).
Relationship between ETCO2 and PaCO2 (a, b) and a Bland-Altman plot showing the bias against average values of ETCO2 and PaCO2 (c, d) in the discriminant function Z > 0 (left panel) and Z < 0 (right panel) groups. ETCO2 was strongly correlated with PaCO2 in the discriminant function Z > 0 group (r2 = 0.7234, p < 0.0001, a). The p-value is < 0.001 in the Z < 0 group as well; however, there is an only weak correlation (r2 = 0.6352) between ETCO2 and PaCO2, respectively (b). The Bland-Altman analysis showed that ETCO2 underestimated PaCO2 by a mean difference (bias) of −3.52 ± 4.86 mmHg (95% CI, −13.0 to 6.00 mmHg) in the Z > 0 group (c) and −8.79 ± 7.29 mmHg (95% CI, −23.1 to 5.50 mmHg) in the Z < 0 group (d).
The Bland–Altman analysis showed that ETCO2 underestimated PaCO2 by a mean difference (bias) of −3.52 ± 4.86 mmHg [95% CI, −13.4 to 6.00] in the Z > 0 group (Fig. 3c) and −8.79 ± 7.29 mmHg [95% CI, −23.07 to 5.50] in the Z < 0 group (Fig. 3d).
Discussion
We found that ETCO2 measured using the mainstream capnometer cap-ONE® was an accurate and reliable noninvasive method for estimating PaCO2 in VLBW infants. The Bland–Altman analysis showed the bias and SD of the differences between ETCO2 and PaCO2 of −5.94 ± 6.63 mmHg. In addition, our results imply that ETCO2 and PaCO2 are likely to correlate better if the tidal volume is larger and the ETT leak is lower; ETCO2 can best be used to estimate PaCO2 in the group with a VT of 8 mL or more and an ETT leak of 7% or less.
Uncuffed ETTs have been the standard of care for pediatric patients under 8 years old based on the presumption that complications such as airway mucosal injury and post-extubation stridor could occur with the use of cuffed ETTs [19, 20].
However, the use of uncuffed ETTs has several disadvantages, including excessive air leakage around the tube leading to unreliable respiratory mechanics, exhaled volumes, and end-expiratory gases, which may be especially important in the intensive care management of intubated pediatric and neonatal patients [21].
The apparatus dead space is of marked interest for capnography because it can lead to the rebreathing of exhaled CO2, thereby causing false inspiratory and expiratory CO2 measurements [22]. Although adapters with a small dead space of about 0.5 mL have become available, the problem regarding dead space remains, especially in preterm infants with low VTs. Furthermore, conventional high sampling flow to measure ETCO2 like 150–200 mL/min using sidestream capnography, underestimates alveolar CO2 concentration in neonatal patients.
ETT leaks reportedly occur in about 70–75% of ventilated infants [23, 24], and the effects of ETT leaks on the measurement of exhaled CO2 are a worrisome problem for pediatric patients, especially for infants. According to the ventilated neonatal lung model study [25], at the end of expiration, when the patient flow is zero, an ETT leak can lead to reverse flow through the adapter, washing out the exhaled CO2 and resulting in an ETCO2 measurement close to zero. Leak-dependent CO2 measurement errors depend on the shape of the CO2 plateau in exhaled air. We previously reported a strong correlation between ETCO2 and PaCO2 when the VT/body weight was 10 mL/kg with a leakage rate of <60% in rabbits [7].
Healthcare providers need to know the minimum VT and maximum ETT leakage values that can be tolerated during ETCO2 measurements in patients with ETT leaks. Greer et al. [26] reported that variations in VT could account for significant differences between ETCO2 and PaCO2. We found that both the VT and the percentage of ETT leak could be used to discriminate the agreement between ETCO2 and PaCO2. When evaluating all measures obtained with a similar degree of ETT leak (for example, 10% of ETT leak), ETCO2 and PaCO2 correlated well with large VTs but not well with smaller VTs. Furthermore, comparing agreement between ETCO2 and PaCO2 when a VT is held, there was a good correlation when the percentage of the ETT leak was small but not well with larger leakage. In particular, the lower the leakage, the more likely ETCO2 and PaCO2 correlate. In terms of the technical methodology, these results are easy to understand and very important.
ETCO2 appears to underestimate PaCO2 values, which could be potentially dangerous. Our results indicate that when estimating the PaCO2 using ETCO2 with a small VT in the presence of relatively small ETT leakage (even ETT leak <7%), the value of ETCO2 might be lower than the actual CO2 concentration, which clinicians need to be aware of.
In situations where the ETCO2 is not correlated with the PaCO2, ETCO2 measurements can be used to confirm the placement of the ET tube in the trachea as long as the additional dead space created by the measurement does not affect the patient’s respiration.
In the presence of ETT leaks, this difference between the displayed and actual VT becomes much more important. According to Vignaux et al., ventilators can underestimate VT in the presence of ETT leak during expiration [27].
In the present study, the VT might not have been accurate because many of the patients had ETT leakage. Besides, we used the VT values obtained from the ventilator; the measurement at the endotracheal tube adapter is more accurate [28]. Furthermore, blood gas measurements and ventilator adjustments were made at the discretion of the team, which innately introduces bias. Moreover, we have in no way accounted for what could potentially be multiple repeated measures in a single subject. Therefore, these issues should be studied in the future.
Conclusion
Our results indicate that ETCO2 is a good surrogate for PaCO2 when VT is high (over 8 mL) and ETT leak is low (<7%). As VT decrees and ETT leak increases, the agreement between the two decreases and reliability of ETCO2 goes down; specifically, ETCO2 underestimates PaCO2.
Although the VTs and the percentage of ETT leaks obtained from the ventilator might not be accurate, such monitoring data could provide additional information for evaluating the accuracy of ETCO2 estimations based on PaCO2 measurements.
References
Erickson SJ, Grauaug A, Gurrin L, Swaminathan M. Hypocarbia in the ventilated preterm infant and its effect on intraventricular haemorrhage and bronchopulmonary dysplasia. J Paediatr Child Health. 2002;38:560–2.
Giannakopoulou C, Korakaki E, Manoura A, Bikouvarakis S, Papageorgiou M, Gourgiotis D, et al. Significance of hypocarbia in the development of periventricular leukomalacia in preterm infants. Pediatr Int. 2004;46:268–73.
Burton GW. The value of carbon dioxide monitoring during anaesthesia. Anaesthesia. 1966;21:173–83.
Bhende MS. End-tidal carbon dioxide monitoring in pediatrics—clinical applications. J Postgrad Med. 2001;47:215–8.
Salthe J, Kristiansen SM, Sollid S, Oglaend B, Soreide E. Capnography rapidly confirmed correct endotracheal tube placement during resuscitation of extremely low birthweight babies (<1000 g). Acta Anaesthesiol Scand. 2006;50:1033–6.
Foy KE, Mew E, Cook TM, Bower J, Knight P, Dean S, et al. Paediatric intensive care and neonatal intensive care airway management in the United Kingdom: the PIC-NIC survey. Anaesthesia. 2018;73:1337–44.
Takahashi D, Hiroma T, Nakamura T. PETCO2 measured by a new lightweight mainstream capnometer with very low dead space volume offers accurate and reliable noninvasive estimation of PaCO2. Res Rep. Neonatol. 2011;1:61–66.
Takahashi D, Matui M, Hiroma T, Nakamura T. A lightweight mainstream capnometer with very low dead space volume is useful monitor for neonates with spontanous and mechanical ventilation; Pilot study. Open J Pediatr. 2012;2:127–32.
Riker JB, Haberman B. Expired gas monitoring by mass spectrometry in a respiratory intensive care unit. Crit Care Med. 1976;4:223–9.
Geiser DR, Rohrbach BW. Use of end-tidal CO2 tension to predict arterial CO2 values in isoflurane-anesthetized equine neonates. Am J Vet Res. 1992;53:1617–21.
Scrivens A, Zivanovic S, Roehr CC. Is waveform capnography reliable in neonates? Arch Dis Child. 2019;104:711–5.
Kugelman A, Golan A, Riskin A, Shoris I, Ronen M, Qumqam N, et al. Impact of continuous capnography in ventilated neonates: a randomized, multicenter study. J Pediatr. 2016;168:e52.
Thompson JE, Jaffe MB. Capnographic waveforms in the mechanically ventilated patient. Respir Care. 2005;50:100–8. discussion 108–9
Tingay DG, Stewart MJ, Morley CJ. Monitoring of end tidal carbon dioxide and transcutaneous carbon dioxide during neonatal transport. Arch Dis Child Fetal Neonatal Ed. 2005;90:F523–526.
Contencin P, Narcy P. Study Group for Neonatology and Pediatric Emergencies in the Parisian Area Size of endotracheal tube and neonatal acquired subglottic stenosis. Arch Otolaryngol Head Neck Surg. 1993;119:815–9.
Deming WE. Statistical adjustment of data. NY: Dover Publications. Wiley; 1943.
Bland JM, Altman DG. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet. 1986;1:307–10.
Hastie T, Tibshirani R, Friedman J. The elements of statistical learning. NY: Springer; 2001.
Motoyama EK, Davis PJ. Smith’s anesthesia for infants and children. 5th ed. St. Louis: CV Mosby, 1990.
Fisher DM. Anesthesia equipment for pediatrics. Pediatric anesthesia, 4th Edn New York: Churchill Livingstone. 2001:207–8.
Main E, Castle R, Stocks J, James I, Hatch D. The influence of endotracheal tube leak on the assessment of respiratory function in ventilated children. Intensive Care Med. 2001;27:1788–97.
Wenzel U, Wauer RR, Wagner MH, Schmalisch G. In vitro and in vivo assessment of the Ventrak 1550/Capnogard 1265 for single breath carbon dioxide analysis in neonates. Br J Anaesth. 1999;83:503–10.
Bernstein G, Knodel E, Heldt GP. Airway leak size in neonates and autocycling of three flow-triggered ventilators. Crit Care Med. 1995;23:1739–44.
Mahmoud RA, Proquitte H, Fawzy N, Buhrer C, Schmalisch G. Tracheal tube airleak in clinical practice and impact on tidal volume measurement in ventilated neonates. Pediatr Crit Care Med. 2011;12:197–202.
Schmalisch G, Al-Gaaf S, Proquitte H, Roehr CC. Effect of endotracheal tube leak on capnographic measurements in a ventilated neonatal lung model. Physiol Meas. 2012;33:1631–41.
Greer KJ, Bowen WA, Krauss AN. End-tidal CO2 as a function of tidal volume in mechanically ventilated infants. Am J Perinatol. 2003;20:447–51.
Vignaux L, Piquilloud L, Tourneux P, Jolliet P, Rimensberger PC. Neonatal and adult ICU ventilators to provide ventilation in neonates, infants, and children: a bench model study. Respir Care. 2014;59:1463–75.
Kim P, Salazar A, Ross PA, Newth CJ, Khemani RG. Comparison of tidal volumes at the endotracheal tube and at the ventilator. Pediatr Crit Care Med. 2015;16:e324–331.
Author information
Authors and Affiliations
Contributions
DT conceptualized and designed the study, designed the data collection instruments, collected data, carried out the initial analyses, drafted the initial manuscript, and reviewed and revised the manuscript. Koko G. designed the data collection instruments, collected data, and reviewed and revised the manuscript. Kei G. conceptualized and designed the study and critically reviewed the manuscript for important intellectual content. All authors approved the final manuscript as submitted and agree to be accountable for all aspects of the work.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflicts of interest to declare.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Takahashi, D., Goto, K. & Goto, K. Effect of tidal volume and end tracheal tube leakage on end-tidal CO2 in very low birth weight infants. J Perinatol 41, 47–52 (2021). https://doi.org/10.1038/s41372-020-0758-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41372-020-0758-2
This article is cited by
-
Relationship between ETCO2 and PaCO2 under Changing Capnogram in Ventilated Infants with NAVA: An Observational Study
Indian Journal of Pediatrics (2023)