Abstract
Aims and objectives
To evaluate the Doppler changes in the intracranial arteries of neonates exposed to perinatal hypoxic insult and compare it with normal neonates.
Materials and methods
Color Doppler of bilateral anterior and middle cerebral arteries was performed within 6 h of birth in 26 healthy neonates and 50 neonates who received delivery room resuscitation (DRR) for perinatal depression and had a 5 min Apgar score <7. Comparisons of resistive index (RI) and peak systolic velocity (PSV) were made between the (a) control group (b) patients with low 5 min Apgar score <7 who without clinical features of neonatal encephalopathy at 24 h (c) neonates with perinatal depression with a clinical evidence of disturbed neurological function at 24 h of birth and examination consistent with mild, moderate, or severe encephalopathy using modified Sarnat and Sarnat’s classification.
Results
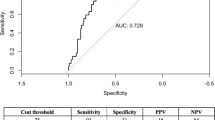
Significantly higher RI was observed in the neonates with to perinatal depression compared to the normal neonates. Significantly higher RI was seen in the patients with clinical features of neonatal encephalopathy (Group C) compared to group B. RI <0.6 and >0.82 was associated with severe neonatal encephalopathy. Differences in PSV were not statistically significant in the various groups.
Conclusion
The study presents the changes in early cerebral Doppler parameters observed in neonates with low 5 min Apgar score following DRR compared to the normal neonates. We also present the relations of Doppler parameters with increasing severity of neonatal encephalopathy according to Sarnat classification.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Badurdeen S, Roberts C, Blank D, Miller S, Stojanovska V, Davis P, et al. Haemodynamic instability and brain injury in neonates exposed to hypoxia- ischaemia. Brain Sci. 2019;9:49.
Kamath-Rayne BD, Jobe AH. Birth asphyxia. Philadelphia, PA, USA: Elsevier; 2016. ISBN 9780323462846.
Kurinczuk JJ, White-Koning M, Badawi N. Epidemiology of neonatal encephalopathy and hypoxic-ischaemic encephalopathy. Early Hum Dev. 2010;86:329–38.
Lawn JE, Lee AC, Kinney M, Sibley L, Carlo WA, Paul VK, et al. Two million intrapartum-related stillbirths and neonatal deaths: where, why, and what can be done? Int J Gynaecol Obstet. 2009;107(Suppl 1):S5–18.
Groenendaal F. Time to start hypothermia after perinatal asphyxia: does it matter? BMJ Paediatr Open. 2019;3:e000494.
Ehrenstein V. Association of Apgar scores with death and neurologic disability. Clin Epidemiol. 2009;1:45–53.
Sarnat HB, Sarnat MS. Neonatal encephalopathy following fetal distress. A clinical and electroencephalographic study. Arch Neurol. 1976;33:696–705.
Queensland Clinical Guidelines. Hypoxic-ischaemic encephalopathy (HIE) clinical guideline education presentation E16.11-1-V3-R21. Queensland Health; Brisbane, Australia. 2018.
Salas J, Tekes A, Hwang M, Northington FJ, Huisman TAGM. Head ultrasound in neonatal hypoxic-ischemic injury and its mimickers for clinicians: a review of the patterns of injury and the evolution of findings overtime. Neonatology. 2018;114:185–97.
Rosenberg AA. Regulation of cerebral blood flow after asphyxia in neonatal lambs. Stroke. 1988;19:239–44.
Polglase GR, Ong T, Hillman NH. Cardiovascular alterations and multiorgan dysfunction after birth asphyxia. Clin Perinatol. 2016;43:469–83. https://doi.org/10.1016/j.clp.2016.04.006.
Hallenbeck JM, Furiow TW. Prostaglandin I; and indometha—cin prevent impairment of post-ischemic brain perfusion in the dog. Stroke. 1979;10:629–37.
Stevens MK, Yaksh TL, Hansen RB, Anderson RE. Effect of preischemic cyclooxygenase inhibition of zomepirac sodium onreflow,cerebral autoregulation, and EEG recovery in the cat after global ischemia. J Cereb Blood Flow Metab. 1986;6:691–702.
Moskowitz MA, Kiwak KJ, Hekimian K, Levine L. Synthesis of compounds with properties of leukotrienes C, and D4 in gerbil brains after ischemia and reperfusion. Science. 1984;224:886–9.
Nishimaki S, Seki K, Yokota S. Cerebral blood flow velocity in two patients with neonatal cerebral infarction. Pediatr Neurol. 2001;24:320–3.
Okumura A, Toyota N, Hayakawa F, Kato T, Maruyama K, Kubota T, et al. Cerebral hemodynamics during early neonatal period in preterm infants with periventricular leukomalacia. Brain Dev. 2002;24:693–7.
Kehrer M, Blumenstock G, Ehehalt S, Goelz R, Poets C, Schöning M. Development of cerebral blood flow volume in preterm neonates during the first two weeks of life. Pediatr Res. 2005;58:927–30.
Liu J, Cao HY, Huang XH, Wang Q. The pattern and early diagnostic value of Doppler ultrasound for neonatal hypoxic-ischemic encephalopathy. J Trop Pediatr. 2007;53:351–4.
Llves P, Talvik R, Talvik T. Changes in Doppler ultrasonography in asphyxiated term infants with hypoxic-ischaemic encephalopathy. Acta Paediatr. 1998;87:680–4.
Guan B, Dai C, Zhang Y, Zhu L, He X, Wang N. Early diagnosis and outcome prediction of neonatal hypoxic-ischemic encephalopathy with color Doppler ultrasound. Diagn Inter Imaging. 2017;98:469–75.
Wu C. The study of cranial ultrasound to diagnostic value of neonatal hypoxic‐ischemic encephalopathy. Ultrasound Obstet Gynecol. 2017;50:107.
Barseem NE, Badr HS, Abdullah MS. Color Doppler ultrasonography in full term neonates with hypoxic ischemic encephalopathy and prediction of outcome. Egypt Pediatr Assoc Gazette. 2016;64:38–43.
Kudrevièienë A, Basevièius A, Lukoðevièius S, Laurynaitienë J, Marmienë V, Nedzelskienë I, et al. The value of ultrasonography and Doppler sonography in prognosticating long-term outcomes among full-term newborns with perinatal asphyxia. Medicina. 2014;50:100–10.
Pinto PS, Tekes A, Singhi S, Northington FJ, Parkinson C, Huisman TA. White-gray matter echogenicity ratio and resistive index: sonographic bedside markers of cerebral hypoxic-ischemic injury/edema? J Perinatol. 2012;32:448–53.
Kumar AS, Chandrasekaran A, Asokan R, Gopinathan K. Prognostic value of resistive index in neonates with hypoxic ischemic encephalopathy. Indian Pediatr. 2016;53:1079–82.
Kirimi E, Tuncer O, Atas B, Sakarya ME, Ceylan A. Clinical value of color Doppler ultrasonography measurements of full-term newborns with perinatal asphyxia and hypoxic ischemic encephalopathy in the first 12 h of life and long-term prognosis. Tohoku J Exp Med. 2002;197:27–33.
Jongeling BR, Badawi N, Kurinczuk JJ, Thonell S, Watson L, Dixon G, et al. Cranial ultrasound as a predictor of outcome in term newborn encephalopathy. Pediatr Neurol. 2002;26:37–42.
Pezzati M, Dani C, Biadaioli R, Filippi L, Biagiotti R, Giani T, et al. Early postnatal doppler assessment of cerebral blood flow velocity in healthy preterm and term infants. Dev Med Child Neurol. 2002;44:745–52.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Kumar, I., Singh, S., Kumar, A. et al. Early postnatal color Doppler changes in neonates receiving delivery room resuscitation with low 5 min Apgar score—a pilot study. J Perinatol 41, 486–493 (2021). https://doi.org/10.1038/s41372-020-00882-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41372-020-00882-x