Abstract
Background
Changes in systemic and cerebral hemodynamics in preterm infants during early transitional circulation are complex and may differ between infants with or without intraventricular hemorrhage (IVH).
Method
In total, 43 infants born at median (range) 25 + 5 (23 + 3–31) had continuous near-infrared spectroscopy (NIRS) monitoring of tissue oxygenation index (TOI) and cerebrovascular reactivity within the first 48 h of life. Measurements of left and right cardiac outputs (LVO, RVO) and patent ductus arteriosus (PDA) were collected at 6, 12, 24, and 48 h of life.
Results
LVO increased within the first 48 h in the IVH (P = 0.007) and no-IVH (P < 0.001) groups. The pattern of change in LVO and RVO was not different between these two groups. TOI was lower in the IVH (P < 0.001) group. A positive correlation between TOI and LVO (P = 0.003) and a negative correlation between the tissue oxygen reactivity index (TOx) and LVO (P = 0.04) were observed at 24 h of life in the IVH group. PDA diameter was not different between IVH groups at any time interval.
Conclusion
Cerebral oxygenation was lower and cerebrovascular reactivity was passive to systemic blood flow at 24 h in infants who developed an IVH.
Similar content being viewed by others
Introduction
Decreased systemic and cerebral blood flow within the first 48 h of life has been associated with the development of an intraventricular hemorrhage (IVH) in preterm infants.1,2,3 The changes in cardiac function and cerebrovascular reactivity within what is described as the “early transitional circulation” have been correlated with the pathophysiology of brain injury in this population.2,3,4,5,6 However, the pattern of changes in systemic and cerebral blood flow and oxygenation and the correlation between them may differ between infants with or without an IVH.
Functional echocardiography and near-infrared spectroscopy (NIRS) have been used as a clinical and research tool to assess changes in systemic and cerebral blood flow and cerebral oxygenation during the transitional circulation in preterm infants.3,7 Measurements of cardiac output flow and ductal patency have been used to guide clinicians on the management of sick preterm infants. However, the correlation between measurements of functional echocardiography and cerebral blood flow and oxygenation remains controversial.
The aim of this study was to explore the changes in systemic and cerebral hemodynamics and assess the complex relationship between these two systems in the mechanisms involved in the possible hypoperfusion–reperfusion injury. We investigated the difference in changes in left and right cardiac outputs and in cerebral oxygenation during the early transitional circulation in infants who develop and did not develop an IVH. We further assessed the correlation between left and right cardiac outputs and cerebral oxygenation and cerebrovascular reactivity for each time interval and for each IVH group independently. In addition, we aimed to describe the hemodynamically significant patent ductus arteriosus (PDA) measurements during the early transitional circulation and their association with IVH over the first 48 h of life.
Methods
This was a prospective observational study, conducted from May 2013 to October 2015 at The Rosie Hospital, Cambridge, UK. The study was authorized by The Research and Development Department of Cambridge University Hospitals NHS Foundation Trust and approved by The East of England Research Ethics Committee (12/EE/0524) in accordance with the declaration of Helsinki. All infants were studied following signed informed parental consent. The initial recruitment protocol was the same applied for previous studies on monitoring of cerebrovascular reactivity recently published by our group, which required continuous measurement of mean arterial blood pressure (MABP). Therefore, only preterm infants born at <32 weeks’ gestational age who had indwelling arterial catheters inserted for clinical reasons and were ≤12 h of life were eligible.8,9 Infants born with major malformations, including cardiac malformations (except for the presence of a foramen ovale or ductus arteriosus) were excluded.
Measurements of cerebral oxygenation
NIRS measurements of tissue oxygenation index (TOI) were collected from a NIRO200NX near-infrared spectrophotometer (Hamamatsu Photonics, KK, Hamamatsu, Japan), using a neonatal NIRS sensor placed on one side of the temporoparietal area of the infants’ head. A sensor holder was used to fix the light source at 3 cm away from the detectors and a lightproof cover was also applied. The sensor was changed to the opposite temporoparietal side of the infant’s head every 6–8 h to avoid any skin marks. This change allowed us to average the data from both sides. The NIRO200NX estimates the TOI by the use of spatially resolved spectroscopy. NIRS measurements were not taken into consideration in the management of the patients by the clinical team, as there was no guideline for the use of cerebral NIRS in preterm infants as routine of care.
Measurements of systemic blood flow and ductus arteriosus patency
Anonymized echocardiographic images were recorded using Vivid S5/Vivid E9 (GE Healthcare) at around 6, 12, 24, and 48 h of life. All measurements were performed retrospectively using EchoPAC clinical workstation software. In order to avoid inter-observer variability, all images and measurements were collected and analyzed by a single clinician (CSdC). The following measurements were performed: (1) Left ventricle output (LVO): aorta diameter was measured from the ascending aorta on the long parasternal view at sinotubular junction (STJ).10 Flow velocity measurement was obtained from a modified five-chamber view. VTI was calculated and averaged from three to five cycles. LVO = stroke volume (π×(aorta diameter)2/4 × VTI)×heart rate/weight.6,11 (2) Right ventricle output (RVO): diameter of the main pulmonary artery was measured from the parasternal view and determined in the end systole at the insertion of the pulmonary valve leaflet just before the valve closes. Flow velocity was performed on the same window, placing the range gate just beyond the valve leaflets and averaged from three to five cycles. RVO = stroke volume (π × (aorta diameter)2/4 × VTI) × heart rate/weight. (3) PDA measurements: diameter was estimated from the 2D image, flow pattern (left to right, right to left, or bidirectional), end-diastolic flow velocity in the LPA (EDF LPA vel) (all from parasternal view), and the presence of reversed diastolic flow in the descending aorta at the abdominal level (RDF DAo) (from high left parasternal view).12,13,14,15 Two senior neonatologists with expertise in cardiology reviewed the anonymized images to confirm structural normality (WK and ZM).
Measurements of physiological data and outcome
Continuous physiological signals of mean arterial blood pressure (MABP), peripheral oxygen saturation (SaO2), and heart rate (HR) were recorded simultaneously from the neonatal intensive care monitors (Marquette Solar 8000 and Carescape B850; GE Healthcare, Milwaukee, Wisconsin). Measurements of transcutaneous carbon dioxide tension (tcpCO2) were collected from a TCM400 monitor (Radiometer). An arterial blood gas was performed before each echocardiography study. The values of hemoglobin (Hb), lactate, pH, partial pressure of oxygen (PaO2), and partial pressure of carbon dioxide (PaCO2) were recorded and included in the analysis. Clinical data within the study period and data on outcome were collected from the medical notes. A separate study chart was kept at the cot side during the study period, which was used by nurses and the research team to record the date and time of events, such as handling/care, examination, procedures, changes on drug infusions, or any other interventions. This allowed us to identify and remove artifacts from the NIRS and physiological signals. Clinical decision-making was at the sole discretion of the clinical team. General information on echocardiography images was shared with the clinical team, such as normal/abnormal anatomy and the presence or absence of a PDA. Measurements of cardiac output and further measurements of ductal significance were not shared with the clinical team, as they were performed retrospectively as batch offline analysis. Cranial ultrasonography scans were performed at the start of the study and repeated every 12–24 h, until the third day of life and then every 1–3 weeks until corrected gestational age at term. IVH was defined according to Papile et al.16 and the greatest grade of hemorrhage during the admission period was used for analysis.
Data analysis
All NIRS and physiological signals were collected and retrospectively analyzed using ICM + software (Cambridge Enterprise Ltd, Cambridge, UK).17 Cerebrovascular reactivity was assessed by the tissue oxygenation heart rate reactivity index (TOHRx), which is the correlation between slow waves of TOI and fluctuations of heart rate (HR), and the tissue oxygen reactivity index (TOx), which is the correlation between TOI and MABP. TOHRx and TOx reflect the reactivity of the cerebral circulation to the changes in the systemic circulation. Zero or negative correlation between TOI and HR or MABP suggests intact cerebrovascular reactivity and positive correlation suggests impaired cerebrovascular reactivity.8,18 TOHRx and TOx were calculated from a moving correlation coefficient, using 5-min time windows between 10-s average values of TOI and HR, and TOI and MABP, respectively. The 5-min window for calculation of TOHRx and TOx has its rationale in the physiology of slow brain waves detected by NIRS (20 s to 3 min).18,19 Tissue oxygen extraction factor (TOEF) was calculated as ((SpO2–TOI)/SpO2). All NIRS, physiological signals, and indices were averaged over 45–60 min before or around the time when echocardiography studies were performed in order to avoid artifacts due to handling or confounders, such as fluid boluses or changes in inotrope/vasopressor infusions.
Statistical analysis
Data distribution was assessed for normality using Shapiro–Wilk test before each statistical test was performed. Independent-samples t test or Mann–Whitney U test were used to investigate the association between mean values of LVO, RVO, PDA measurements, NIRS measurements, and IVH. Chi-square test was used to compare groups with dichotomous variables. Pearson correlation coefficient or Spearman correlation was used to assess the relationship between functional echocardiographic measurements and cerebral oxygenation and cerebrovascular reactivity measurements. ANOVA with Bonferroni correction and Kruskall–Wallis test was applied for multiple comparisons between mean values of cardiac outputs and cerebral oxygenation between time intervals. Statistical analysis was done using SPSS software package version 25 (SPSS, Inc., Chicago, IL, USA). To provide prediction models for the differentiation between patients with or without IVH concerning the pattern of changes across measurements, the relationships between the variables of interest and the different measurement time points were expressed as linear mixed effect models (R package lme4). As fixed effects, we entered the variable of interest and the patient group (condition status: IVH and no IVH) into the model. As random effects, we had intercepts for the repeated measurement points for each patient. The chi-square and the P-values for comparison of the models were obtained by likelihood ratio tests of the full model, with random intercepts against the null model without random intercepts. A statistical test was considered significant if the P value was <0.05 (two tailed).
Results
A total of 50 infants were recruited; however, two infants were diagnosed with cardiac malformations: one infant had a ventricle septum defect (VSD) and another had a double superior vena cava (SVC). One infant had myocardial hypertrophy and developed a pericardial effusion after 24 h of life. Four infants were diagnosed with persistent pulmonary hypertension of the newborn (PPHN), requiring nitric oxide (NO). Data from these seven infants were excluded from the data analysis, as measurements of flow during the early physiological transitional circulation could be biased. Nineteen infants did not have echocardiographic measurements at 6 h of life, because they were recruited around 12 h of life. Nine infants did not have echocardiographic measurements at 12, 24, or 48 h of life, either due to clinical deterioration at the time of the scan or because CSdC was not available to perform the scans.
Table 1 shows the characteristics of all infants included in the data analysis. All of them were mechanically ventilated at the start of data collection. Three infants required high-frequency oscillatory ventilation (7%) within the first 28 h of life; the remaining infants received volume target ventilation. Eighteen (42%) infants were extubated and commenced on biphasic or conventional continuous positive airway pressure. None of the infants died before 48 h of age. Infants died from complications of extreme prematurity, such as fulminant NEC, sepsis, or multi-organ failure. Fourteen infants developed an IVH; six (43%) of them were classified as severe IVH (grade III or IV) and eight (57%) were classified as mild/moderate IVH (grade II). Ten (71%) infants had an IVH diagnosed at or after 24 h of life and all IVHs were diagnosed within the first 3 days of life. Only one infant received treatment for PDA during the study (around 36 h of life). This infant received an incomplete course of ibuprofen in view of clinical deterioration and died just after 48 h of life. The median (range) age at the start of PDA treatment was 13 (2–63) days of life. Seventeen (39.5%) infants received treatment for their PDA with either ibuprofen (N = 13) or paracetamol (N = 4). One infant had the PDA surgically ligated at 63 days of life and had not received treatment with medication prior to ligation.
Changes in left and right cardiac output and cerebral oxygenation in the first 48 h of life
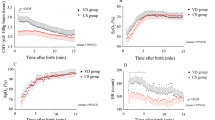
Figure 1a shows the mean LVO for each time interval for the IVH and no-IVH groups. Infants who did no develop an IVH had a statistically significant increase in mean LVO over the first 48 h of life (P < 0.001). Although mean LVO seems to have a steadier increase in the no-IVH group compared with infants in the IVH group and the mean LVO appears lower at 12 h and higher at 24 h in the IVH group compared with no-IVH group, there was no significant statistical difference between groups (Table 2). Using mixed model analysis to compare the pattern of change in LVO between infants who had and did not have an IVH, no significant difference was observed (P = 0.66) for full model analysis.
Changes in left and right cardiac output and TOI and TOEF within the first 48 h of life. Mean left ventricle output (LVO) had a significant increase in the no-IVH (P < 0.001) and IVH (P = 0.007) groups. Mean right ventricle output (RVO) had a significant increase in the no-IVH group (P < 0.001) but no significant increase in the IVH group (P = 0.203). Mean tissue oxygenation index (TOI) had no significant increase in either no-IVH (P = 0.38) or IVH (P = 0.85) groups. Mean tissue oxygen extraction factor (TOEF) had a significant increase in the no-IVH group (P = 0.07) but no significant increase in the IVH group (P = 0.86). One-way ANOVA
Figure 1b shows the mean RVO for each time interval for IVH and no-IVH groups. The mean RVO showed a statistically significant increase over the first 48 h in the no-IVH group (P < 0.001) but not in the IVH group (P = 0.20). Using mixed model analysis, the patterns of change in RVO between IVH groups showed no statistical difference (P = 0.71) for full model analysis.
The mean TOI and mean TOEF did not have a statistically significant increase or decrease over the first 48 h of life in either the IVH or no-IVH groups. However, the mixed model analysis showed that the patterns of change in TOI between these two groups were statistically different (P < 0.001) for full model analysis. The difference in the pattern of changes in TOEF between groups did not reach statistical significance (P = 0.066), for full model analysis. Mean TOI was significantly lower at 12, 24, and 48 h in the IVH group compared with the no-IVH group, and mean TOEF was significantly higher between these two groups in those same time intervals (Table 2).
Mean TOx and mean TOHRx had no difference within the first 48 h in the no-IVH group (P = 0.65 and P = 0.87, respectively) or in the IVH group (P = 0.54 and P = 0.61). Mixed model analysis showed no difference between patterns of changes in TOx (P = 0.57) or TOHRx (P = 0.71) between the IVH groups, for full model analysis.
Correlation between left and right cardiac outputs and cerebral oxygenation and cerebrovascular reactivity
Positive correlations between LVO and RVO and TOI were observed at 24 h of life in infants who developed an IVH, suggesting passivity of cerebral blood flow to changes in systemic blood flow. A significant negative correlation between TOx and LVO was observed at 24 h of life in this same group; this suggests that the lower the systemic blood flow, the more impaired cerebral autoregulation (Table 3). TOHRx had no significant correlation with LVO or RVO (Table 3). When only infants with PDA >1.5 mm were included in the analysis (N = 9), a significant negative correlation between TOHRx and RVO was observed (r = −0.741, P = 0.022), but the correlation between TOHRx and LVO did not reach statistical significance (r = −0.611, P = 0.08).
No other significant correlations between cardiac output and TOI, TOx, or TOHRx were observed in any other time intervals neither in the IVH nor in the no-IVH groups.
Changes in PDA measurements in the first 48 h of life
Table 4 shows the characteristics of the PDA measurements at 6, 12, 24, and 48 h. PDA size did not significantly change over the first 48 h of life, but other measurements of hemodynamic significance, such as the presence of reversed end-diastolic flow in the abdominal aorta (RDF DAo), LA:Ao ratio, and end-diastolic flow velocity in the LPA (EDF LPA vel), increased over time. By 48 h of life, the flow pattern changed to bidirectional with mainly left-to-right flow or pulsatile. In our cohort, only a small number of infants had a constricting or closing PDA.
There was no difference in measurements of PDA 2D diameter between infants who had and did not have an IVH at any time interval (Table 2). EDF LPA vel was higher in infants who did not have an IVH compared with those who had an IVH at 12 and 24 h of life (Table 2). The percentage of infants who had the presence of RDF DAo was higher in the no-IVH group compared with the IVH group at 24 h of life (Table 2).
There was no difference in Hb at 6-h (P = 0.27) or 24-h (P = 0.24) intervals. Hb (g/L) (mean, 95% CI) tended to be significantly lower at 12 h of the IVH group (136, 124–148) compared with the no-IVH group (153, 143–163), P = 0.06. In contrast, Hb (g/L) (mean, 95% CI) was significantly higher in the IVH (141, 132–150) compared with the no-IVH group (125, 113–137) at 48 h (P = 0.02). There was no difference in PaCO2 (P = 0.82), pH (P = 0.59), or lactate (P = 0.90) between IVH groups at 24 h or at any other interval.
Discussion
This study described the relationship between systemic blood flow and cerebral oxygenation and cerebrovascular reactivity, as well as the changes in PDA measurements, within the first 48 h of life in preterm infants who did and did not develop an IVH.
Cardiac output increased significantly over the first 48 h of life in infants who did not develop an IVH, and the mean (range) values of LVO and RVO for this group were similar to data previously published on “normal” thresholds for cardiac output during the transitional circulation in stable preterm infants.6,11,20 However, in contrast, our cohort consisted mainly of preterm infants who were ventilated for the majority of time the data were collected and half of whom were started on inotropes or vasopressors within the study period. The mean LVO and mean RVO over the first 48 h of life were not different between the IVH groups. Noori et al. (2014), using a similar cohort of preterm infants and study design, did not find a difference between the mean LVO in the first 72 h of life in infants with or without IVH. However, they showed that the pattern of changes in LVO tended to be different between these two groups.3 In our cohort, although the pattern of changes in LVO within the first 48 h of life showed no statistical difference, they were similar to those results described by Noori et al. (2014). In infants who developed an IVH, we observed that LVO may be lower around 12 h of life, which was followed by a significant increase at 24 h of age to levels above the mean LVO in the group of infants who did not develop an IVH. Noori et al. (2014) described this pattern of change in LVO as consistent with hemodynamic changes associated with a hypoperfusion–reperfusion cycle and, as similar to our data, the increase in LVO was observed just before the IVH occurred (around or soon after 24 h of life for our cohort).
The mean TOI was significantly lower and the mean TOEF was significantly higher from 12 h of life in infants who developed an IVH. Several studies have shown lower cerebral oxygenation with higher oxygen consumption in infants who had brain injury.3,21 Most of these studies included only infants with severe IVH; however, in our cohort, more than half of the infants developed moderate IVH (grade II), suggesting that even a low/moderate-grade IVH could be associated with distant effects of cerebral oxygenation and cerebrovascular reactivity, since cerebral NIRS measures the changes in oxygenated and deoxygenated Hb in the cortical and subcortical areas of the brain. In addition, our study demonstrated that the pattern of changes in the TOI was statistically different between infants who had and did not have an IVH. Infants who did not develop an IVH seemed to follow a steady increase in the TOI from 6 h of life, while infants who had an IVH showed a decrease in the TOI from 6 to 12 h of life, and the levels remained lower in this group compared with the no-IVH group throughout 48 h.
The use of a correlation coefficient between continuous NIRS, with static measurements of systemic blood flow, has been applied in several studies, but the results remain controversial.22,23,24 Most studies perform group analysis, with no discrimination between infants who had or did not have an IVH.24,25,26 We observed a positive correlation between the TOI and LVO and RVO, and a negative correlation between TOx and LVO, at the 24-h interval in infants who developed an IVH, suggesting that cerebral blood flow became passive to systemic blood flow only in infants who developed the injury. Because most infants in our cohort developed an IVH just before or after 24 h of life, these correlations between systemic and cerebral blood flow may reflect the loss of cerebral autoregulation and cerebrovascular reactivity during the reperfusion injury or during the active hemorrhage. The evidence that changes in cardiac output affect cerebral blood flow is controversial. It has been suggested that cerebral blood flow is controlled based on integrating mechanisms that would take into account changes in cardiac output. It is already known from basic physiology that optimal organ perfusion is dependent on the relationship between blood pressure and specific organ vascular resistance and that the distribution of cardiac output is dependent on the organ’s metabolic requirement.27 There is evidence that dynamic cerebral autoregulation is not affected by acute changes in cardiac output; however, it remains unknown if the plateau or upper and lower limits of autoregulation are changed when cardiac output is altered, particularly in infants at risk to develop and with established brain injury. Studies in adults have shown that cerebral blood flow may either be independent or passive to changes in cardiac output.28,29,30,31 In adult patients with stroke, an association between cardiac output and cerebral blood flow velocity (estimated using transcranial Doppler) was present in the areas affected with ischemia but not in the unaffected areas.32 Moreover, there is evidence that cardiac output contributes to the regulation of cerebral blood flow via sympathetic nervous system.33 In neonates, there is not much evidence that changes in cardiac output will result in changes in cerebral blood flow. A short report from Kusaka et al. (2005) has shown a positive correlation between cardiac output and cerebral blood flow in a small cohort of infants undergoing intensive care.34 It has been suggested that cardiac output would be regulated by changes in heart rate in preterm infants as a compensation for the immature myocardium.35 In our study, we only observed a positive correlation between cerebral blood flow and cardiac output in infants who had an IVH and this correlation was only present around the possible time that reperfusion or the injury occurred. It is possible that the regulatory mechanism may have been disrupted at this time, and that the cerebral circulation in those infants with a susceptibility to develop an IVH may be passive to changes in systemic hemodynamics. However, it will always be difficult to be precise as to the exact timing of the start of the injury.
In studies assessing the relationship between cardiac output and cerebral blood flow, the presence of a PDA could be a confounding factor. In the presence of a hemodynamically significant PDA (>1.5 mm and the presence of left-to-right flow), LVO would reflect the pulmonary blood flow and RVO the systemic blood flow. Noori et al.3 argued that the differences in LVO patterns in infants with or without an IVH could be related to changes in the PDA rather than systemic blood flow. In our cohort, >50% of the infants had a PDA above 1.5 mm before 48 h of life. However, other markers of hemodynamic significance and “ductal steal” such as an increased LA:Ao, the presence of reversal flow on the post-ductal aorta, high LPA end- diastolic flow velocity, and high LVO, increased over the time intervals, and became more significant after 24 h. Moreover, there was no difference in the LA:Ao ratio and LVO between groups, whereas mean EDF velocity in the LPA and the presence of reverse EDF in the post-ductal aorta were higher in infants who did not develop an IVH. Therefore, it is possible that, within the first 24 h of life, LVO may still be a reliable trend measurement of systemic blood flow and consequently ductal steal may not affect cerebral blood flow.
The relationship between PDA and IVH may be complex and possibly multifactorial. In our study, no association was observed between measurements of hemodynamic significant PDA and IVH. Studies reporting that preterm infants who developed an IVH had a larger PDA than those who did not develop an IVH, have shown limitations, such as PDA measurements, which were collected after the development of the IVH and in some old studies, the lack of antenatal steroids.36,37 In our study, nearly 100% of the infants received antenatal steroids. Moreover, most PDA measurements were performed before the IVH was detected on cranial ultrasound.
Limitations
The comparison between continuous NIRS measurements with static functional echocardiographic measurements is an important limitation in our study. There is no consensus on how to average NIRS data for correlations with cardiac data. We averaged the NIRS data around the 1-h interval just before or around the echocardiography measurements, always trying to avoid periods in which drugs or transfusions were given or inotropes and vasopressors were increased or decreased, as they may affect cardiac output measurements. Other studies have applied NIRS data averaged only before or only during echocardiographic measurements.3,24,25
Our cohort was small, and our study was not powered to include multivariate analysis, including several other factors related to development of low systemic blood flow and changes in cerebral oxygenation associated with IVH. The immature myocardium has been claimed as one of the major etiological factors related to the decreased cardiac output and low cerebral blood flow in preterm infants following birth.38 However, changes in systemic and cerebral hemodynamics may be influenced by a combination of different factors, such as peripheral vascular resistance, end-organ metabolism regulation, autonomic response, and genetic predisposition to IVH.
In our study, the mean Hb was lower at 12 h of life in the IVH group. This could reflect the initial bleeding in those infants who had an IVH diagnosed at the scan performed around 24 h of life and may have had an influence in the lower TOI and LVO observed at 12 h of age. Interestingly, Hb was significantly higher at 48 h of life in those who developed an IVH, which may have been related to red cells transfusions post hemorrhage. In addition, changes in CO2 may disrupt cerebral autoregulatory mechanisms by causing vasoconstriction or vasodilation, and therefore compromise the data on cerebrovascular reactivity. However, in our data, PaCO2 had no difference between IVH and no-IVH groups.
Neonatal functional echocardiography may be a useful tool to assess changes in systemic blood flow and ductal patency in preterm infants during the transitional circulation. However, the lack of correlation between measurements of LVO, RVO, and PDA with NIRS signals of cerebral oxygenation and cerebrovascular reactivity during most of the recording time may demonstrate the difficulties in assessing changes between systemic and cerebral hemodynamics using static versus continuous measurements. The relationship between systemic blood flow and cerebrovascular reactivity may be better understood by using measurements of continuous cardiac output.39,40 However, further work is required to validate this technique in the preterm population, taking into account the challenges posed by the ductal and atrial shunts.
Conclusion
Cerebral oxygenation was lower in infants who developed an IVH and loss of cerebrovascular reactivity was present just before or during the hemorrhage. The significant correlation between systemic and cerebral blood flow observed only at 24 h of life in infants who developed an IVH may reflect the impaired cerebrovascular reactivity during hypoperfusion and reperfusion cycle.
References
Meek, J. H. et al. Low cerebral blood flow is a risk factor for severe intraventricular haemorrhage. Arch. Dis. Child Fetal Neonatal Ed. 81, F15–F18 (1999).
Kluckow, M. & Evans, N. Low superior vena cava flow and intraventricular haemorrhage in preterm infants. Arch. Dis. Child Fetal Neonatal Ed. 82, F188–F194 (2000).
Noori, S. et al. Changes in cardiac function and cerebral blood flow in relation to peri/intraventricular hemorrhage in extremely preterm infants. J. Pediatr. 164, 264–270 (2014). e261-263.
Soul, J. S. et al. Fluctuating pressure-passivity is common in the cerebral circulation of sick premature infants. Pediatr. Res. 61, 467–473 (2007).
Pryds, O. et al. Heterogeneity of cerebral vasoreactivity in preterm infants supported by mechanical ventilation. J. Pediatr. 115, 638–645 (1989).
Evans, N. & Kluckow, M. Early determinants of right and left ventricular output in ventilated preterm infants. Arch. Dis. Child Fetal Neonatal Ed. 74, F88–F94 (1996).
Wong, F. Y. et al. Impaired autoregulation in preterm infants identified by using spatially resolved spectroscopy. Pediatrics 121, e604–e611 (2008).
da Costa, C. S. et al. Monitoring of cerebrovascular reactivity for determination of optimal blood pressure in preterm infants. J. Pediatr. 167, 86–91 (2015).
da Costa, C. S. et al. Optimal mean arterial blood pressure in extremely preterm infants within the first 24 h of life. J. Pediatr. 203, 242–248 (2018).
Beker, F. et al. Echocardiographic assessment of left ventricular outflow tract diameter in preterm infants. Austral. J. Ultrasound Med. 17, 146–149 (2014).
Mandelbaum-Isken, V. H. & Linderkamp, O. Cardiac output by pulsed Doppler in neonates using the apical window. Pediatr. Cardiol. 12, 13–16 (1991).
Su, B. H. et al. Echocardiographic assessment of patent ductus arteriosus shunt flow pattern in premature infants. Arch. Dis. Child Fetal Neonatal Ed. 77, F36–F40 (1997).
Kluckow, M. & Evans, N. Early echocardiographic prediction of symptomatic patent ductus arteriosus in preterm infants undergoing mechanical ventilation. J. Pediatr. 127, 774–779 (1995).
El Hajjar, M. et al. Severity of the ductal shunt: a comparison of different markers. Arch. Dis. Child Fetal Neonatal Ed. 90, F419–F422 (2005).
Groves, A. M., Kuschel, C. A., Knight, D. B. & Skinner, J. R. Does retrograde diastolic flow in the descending aorta signify impaired systemic perfusion in preterm infants? Pediatr. Res. 63, 89–94 (2008).
Papile, L. A. et al. Incidence and evolution of subependymal and intraventricular hemorrhage: a study of infants with birth weights less than 1500 gm. J. Pediatr. 92, 529–534 (1978).
Smielewski, P. et al. ICM + : software for on-line analysis of bedside monitoring data after severe head trauma. Acta Neurochir. Suppl. 95, 43–49 (2005).
Mitra, S. et al. Heart rate passivity of cerebral tissue oxygenation is associated with predictors of poor outcome in preterm infants. Acta Paediatr. 103, e374–e382 (2014).
Brady, K. M. et al. Continuous time-domain analysis of cerebrovascular autoregulation using near-infrared spectroscopy. Stroke 38, 2818–2825 (2007).
Walther, F. J. et al. Pulsed Doppler determinations of cardiac output in neonates: normal standards for clinical use. Pediatrics 76, 829–833 (1985).
Alderliesten, T. et al. Cerebral oxygenation, extraction, and autoregulation in very preterm infants who develop peri-intraventricular hemorrhage. J. Pediatr. 162, 698–704 e692 (2013).
Kissack, C. M. et al. Cerebral fractional oxygen extraction in very low birth weight infants is high when there is low left ventricular output and hypocarbia but is unaffected by hypotension. Pediatr. Res. 55, 400–405 (2004).
Victor, S. et al. The relationship between cardiac output, cerebral electrical activity, cerebral fractional oxygen extraction and peripheral blood flow in premature newborn infants. Pediatr. Res. 60, 456–460 (2006).
Sirc, J., Dempsey, E. M. & Miletin, J. Cerebral tissue oxygenation index, cardiac output and superior vena cava flow in infants with birth weight less than 1250 grams in the first 48 h of life. Early Hum. Dev. 89, 449–452 (2013).
Takami, T. et al. Changes in cerebral perfusion in extremely LBW infants during the first 72 h after birth. Pediatr. Res. 68, 435–439 (2010).
Moran, M. et al. Cerebral tissue oxygenation index and superior vena cava blood flow in the very low birth weight infant. Acta Paediatr. 98, 43–46 (2009).
Williams, L. R. & Leggett, R. W. Reference values for resting blood flow to organs of man. Clin. Phys. Physiol. Meas. 10, 187–217 (1989).
Levine, B. D. et al. Cerebral versus systemic hemodynamics during graded orthostatic stress in humans. Circulation 90, 298–306 (1994).
Ogoh, S. et al. The effect of changes in cardiac output on middle cerebral artery mean blood velocity at rest and during exercise. J. Physiol. 569, 697–704 (2005).
Choi, B. R. et al. Factors associated with decreased cerebral blood flow in congestive heart failure secondary to idiopathic dilated cardiomyopathy. Am. J. Cardiol. 97, 1365–1369 (2006).
Berre, J. et al. Dobutamine increases cerebral blood flow velocity and jugular bulb hemoglobin saturation in septic patients. Crit. Care Med. 25, 392–398 (1997).
Meng, L. et al. Cardiac output and cerebral blood flow: the integrated regulation of brain perfusion in adult humans. Anesthesiology 123, 1198–1208 (2015).
Cencetti, S. et al. Autonomic control of the cerebral circulation during normal and impaired peripheral circulatory control. Heart 82, 365–372 (1999).
Kusaka, T. et al. Cerebral distribution of cardiac output in newborn infants. Arch. Dis. Child Fetal Neonatal Ed. 90, F77–F78 (2005).
Noori S. S. T., Seri I. Neonatology Questions and Controversies: Hemodynamics and Cardiology (ed. Kleinman C. S. I.) 3–22 (Elsevier Sauders, Philadelphia, USA, 2012).
Evans, N. & Kluckow, M. Early ductal shunting and intraventricular haemorrhage in ventilated preterm infants. Arch. Dis. Child Fetal Neonatal Ed. 75, F183–F186 (1996).
Jim, W. T. et al. Cerebral hemodynamic change and intraventricular hemorrhage in very low birth weight infants with patent ductus arteriosus. Ultrasound Med. Biol. 31, 197–202 (2005).
Osborn, D. A., Evans, N. & Kluckow, M. Left ventricular contractility in extremely premature infants in the first day and response to inotropes. Pediatr. Res. 61, 335–340 (2007).
Ballestero, Y. et al. Measurement of cardiac output in children by bioreactance. Pediatr. Cardiol. 32, 469–472 (2011).
Weisz, D. E. et al. Non-invasive cardiac output monitoring in neonates using bioreactance: a comparison with echocardiography. Neonatology 102, 61–67 (2012).
Financial support
SPARKS charity (11CUH02); Cambridge Trust and Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES) (PhD scholarship to Dr. Sortica da Costa/9418–11–3).
Author information
Authors and Affiliations
Contributions
Substantial contributions to conception and design of the research work: C.S.da.C., Z.M., M.C., P.S. and T.A. Acquisition of data or analysis, and interpretation of data: C.S.da.C., D.C., I.Ng., Z.M. and W.K. Drafting the article: C.S.da.C. Revising the article critically for important intellectual content: D.C., Z.M., I.Ng., W.K., M.C., P.S. and T.A. Final approval of the version to be published: all authors.
Corresponding author
Ethics declarations
Competing interests
The ICM+® software (ICM+; www.neurosurg.cam.ac.uk/icmplus) used for data monitoring and analysis is licensed by the Cambridge Enterprise Limited (University of Cambridge). Dr. Peter Smielewski and Professor Marek Czosnyka have an interest in a fraction of the licensing fee. The other authors have no conflicts of interests to disclosure.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Sortica da Costa, C., Cardim, D., Molnar, Z. et al. Changes in hemodynamics, cerebral oxygenation and cerebrovascular reactivity during the early transitional circulation in preterm infants. Pediatr Res 86, 247–253 (2019). https://doi.org/10.1038/s41390-019-0410-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41390-019-0410-z
This article is cited by
-
The Role of Oxidative Stress in the Progression of Secondary Brain Injury Following Germinal Matrix Hemorrhage
Translational Stroke Research (2023)
-
Near-infrared spectroscopy monitoring of neonatal cerebrovascular reactivity: where are we now?
Pediatric Research (2023)
-
Neuromonitoring in neonatal critical care part II: extremely premature infants and critically ill neonates
Pediatric Research (2023)
-
A neonatal neuroNICU collaborative approach to neuromonitoring of posthemorrhagic ventricular dilation in preterm infants
Pediatric Research (2022)
-
Clinical determinants of cerebrovascular reactivity in very preterm infants during the transitional period
Pediatric Research (2022)