Abstract
Background
Preterm very-low-birth-weight (≤1500 g) infants exhibit disproportionate weight-for-length growth in the Neonatal Intensive Care Unit.
Local problem
High frequency of body mass index (BMI) > 90th centile at discharge and 1-year postnatal age associated with elevated blood pressure and serum leptin in infancy and adolescence.
Methods
Single-institution quality improvement project in appropriately grown infants born at 230/7–286/7 weeks gestational age and discharged home.
Intervention
Adjustable feeding protocol based on valid serial length measurements (board or caliper).
Results
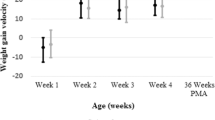
The average monthly percentage of weight-for-length disproportion at discharge decreased from 13% in Epoch 1 to 0% in Epoch 2 (P < 0.05). Although the average Z-score for BMI at discharge was lower in Epoch 2 versus Epoch 1 (P < 0.01), this was absent by 1 year follow-up (P = 0.91).
Conclusions
Adjustable feedings plus use of accurate serial length measurements decreases weight-for-length disproportion at hospital discharge but not at 1 year.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
Change history
10 October 2019
An amendment to this paper has been published and can be accessed via a link at the top of the paper.
References
Barker DJ, Osmond C, Golding J, Kuh D, Wadsworth ME. Growth in utero, blood pressure in childhood and adult life, and mortality from cardiovascular disease. Br Med J. 1989;298:564–7.
Barker DJ. The developmental origins of adult disease. J Am Coll Nutr. 2004;23(Suppl. 6):588S–95.
Potenza MV, Mechanick JI. The metabolic syndrome: definition, global impact, and pathophysiology. Nutr Clin Pract. 2009;24:560–77.
Reinehr T, Kleber M, Toschke AM. Small for gestational age status is associated with metabolic syndrome in overweight children. Eur J Endocrinol. 2009;160:579–84.
Parkinson JR, Hyde MJ, Gale C, Santhakumaran S, Modi N. Preterm birth and the metabolic syndrome in adult life: a systematic review and meta-analysis. Pediatrics. 2013;131:e1240–63.
Skilton MR, Viikari JS, Juonala M, Laitinen T, Lehtimäki T, Taittonen L, et al. Fetal growth and preterm birth influence cardiovascular risk factors and arterial health in young adults: the Cardiovascular Risk in Young Finns Study. Arterioscler Thromb Vasc Biol. 2011;31:2975–81.
Kerkhof GF, Breukhoven PE, Leunissen RW, Willemsen RH, Hokken-Koelega AC. Does preterm birth influence cardiovascular risk in early adulthood? J Pediatr. 2012;161:390–1.
Kaseva N, Vääräsmäki M, Matinolli H-M, Sipola-Leppänen M, Tikanmäki M, Heinonen K, et al. Pre-pregnancy overweight or obesity and gestational diabetes as predictors of body composition in offspring twenty years later: evidence from two birth cohort studies. Int J Obes. 2018;42:872–9.
Catalano PM, Shankar K. Obesity and pregnancy: mechanisms of short term and long term adverse consequences for mother and child. Brit Med J. 2017;360:j1.
Khalak R, Rijhsinghani A, McCallum SE. Impact of maternalobesity on very preterm infants. Obesity. 2017;25:945–9.
Fabricius-Bjerre S, Jensen RB, Færch K, Larsen T, Mølgaard C, Michaelsen KF, et al. Impact of birth weight and early infant weight gain on insulin resistance and associated cardiovascular risk factors in adolescence. PLoS ONE. 2011;6:e20595.
Elks CE, Loos RJ, Sharp SJ, Langenberg C, Ring SM, Timpson NJ, et al. Genetic markers of adult obesity risk are associated with greater early infancy weight gain and growth. PLoS Med. 2010;7:e1000284.
Perng W, Rifas-Shiman SL, Kramer MS, Haugaard LK, Oken E, Gillman MW, et al. Early weight gain, linear growth, and mid-childhood blood pressure. a prospective study in project viva. Hypertension. 2016;67:301–8.
Kerkhof GF, Leunissen RW, Hokken-Koelega AC. Early origins of the metabolic syndrome: role of small size at birth, early postnatal weight gain, and adult IGF-I. J Clin Endocrinol Metab. 2012;97:2637–43.
Duncan AF, Heyne RJ, Morgan JS, Ahmad N, Rosenfeld CR. Elevated systolic blood pressure in preterm very-low-birth-weight infants ≤ 3 years of life. Pediatr Nephrol. 2011;26:1115–21.
Frankfurt JA, Duncan AF, Heyne RJ, Rosenfeld CR. Renal function and systolic blood pressure in very-low-birth-weight infants 1-3 years of age. Pediatr Nephrol. 2012;27:2285–91.
Duncan AF, Frankfurt JA, Heyne RJ, Rosenfeld CR. Biomarkers of adiposity are elevated in preterm very-low-birth-weight infants at 1, 2 and 3 Y of age. Pediadtr Res. 2017;81:780–6.
Pavageau L, Whitham J, Rosenfeld CR, Heyne RJ, Brown LS, Brion LP. Valid serial length measurements in preterm infants permit characterization of growth patterns. J Perinatol 2018;38:1694–1670.
Olsen IE, Groveman SA, Lawson ML, Clark RH, Zemel BS. New intrauterine growth curves based on United States data. Pediatrics. 2010;125:e214–24.
Sanchez AM, Mize SG, Jimenez JM, Manroe BL, Rosenfeld CR, Tyson TE. Systems approach to the evaluation of maternal and neonatal care. Proc 12th Hawaii Int’l Conf Syst Sci, Sel Pap Med Inf Process. 1979;3:140–51.
Fenton TR, Kim JH. A systematic review and meta-analysis to revise the Fenton growth chart for preterm infants. BMC Pediatr. 2013;13:59.
WHO Multicentre Growth Reference Study Group. WHO Child Growth Standards based on length/height, weight and age. Acta Paediatr. 2006;450:76.
Holston A, Stokes T, Olsen C, Choi YS, Curtis J, Higginson J, et al. Novel noninvasive anthropometric measure in preterm and full-term infants: normative values for waist circumference:length ratio at birth. Pediatr Res. 2013;74:299–306.
Rochow N, Raja P, Liu K, Fenton T, Landau-Crangle E, Göttler S, et al. Physiological adjustment to postnatal growth trajectories in healthy preterm infants. Pedia Res. 2016;79:870–9.
Olsen IE, Lawson ML, Ferguson AN, Cantrell R, Grabich SC, Zemel BS, et al. BMI curves for preterm infants. Pediatrics. 2015;135:e572–e581.
Daly-Wolfe KM, Jordan KC, Slater H, Beachy JC, Moyer-Mileur LJ. Mid-arm circumference is a reliable method to estimate adiposity in preterm and term infants. Pedia Res. 2015;78:336–41.
Vaucher YE, Harrison GG, Udall TN, Morrow G III. Skinfold thickness in North American Infants 24–41 weeks gestation. Hum Biol. 1984;56:713–31.
Abdel-Rahman SM, Paul IM, Delmore P, James L, Fearn L, Atz AM, et al. Best Pharmaceuticals for Children Act – Pediatric Trials Network. An anthropometric survey of US pre-term and full-term neonates. Ann Hum Biol. 2017;44:678–86.
WHO Multicentre Growth Reference Study Group. WHO child growth standards: length/height-for-age, weight-for-age, weight-for-length, weight-for-height and body mass index-for-age: methods and development. Geneva: World Health Organization; 2006. https://www.who.int/childgrowth/standards/Technical_report.pdf. Accessed 12 Feb 2018.
WHO Multicentre Growth Reference Study Group. WHO child growth standards: head circumference-for-age, arm circumference-for-age, triceps skinfold-for-age and subscapular skinfold-for-age: methods and development. Geneva: World Health Organization;; 2007. https://www.who.int/childgrowth/standards/second_set/technical_report_2.pdf. Accessed 12 Feb 2018.
Patel AL, Engstrom JL, Meier PP, Jegier BJ, Kimura RE. Calculating postnatal growth velocity in very low birth weight (VLBW) premature infants. J Perinatol. 2009;29:618–22.
Landau-Crangle E, Rochow N, Fenton TR, Liu K, Ali A, So HY. et al. Individualized postnatal growth trajectories for preterm infants. JPEN J Parent Enter Nutr. 2018;42:1084–92.
Kaiser JR, Bai S, Gibson N, Holland G, Lin TM, Swearingen CJ, et al. Association between transient newborn hypoglycemia and fourth-grade achievement test proficiency: a population-based study. JAMA Pediatr. 2015;169:913–21.
McKinlay JCD, Alsweiler JM, Anstice NS, Burakevych N, Chakraborty A, Chase JG, et al. Children with hypoglycemia and their later development (chyld) study team, association of neonatal glycemia with neurodevelopmental outcomes at 4.5 years. JAMA Pediatr. 2017;171:972–83.
Thornton PS, Stanley CA, De Leon DD, Harris D, Haymond MW, Hussain K, et al. Pediatric endocrine society, recommendations from the pediatric endocrine society for evaluation and management of persistent hypoglycemia in neonates, infants, and children. J Pediatr. 2015;167:238–45.
Kliegman RM, Walsh MC. Neonatal necrotizing enterocolitis: pathogenesis, classification, and spectrum of illness. Curr Probl Pediatr. 1987;17:213–88.
Cooke RJ, Griffin I. Altered body composition in preterm infants at hospital discharge. Acta Pædiatr. 2009;98:1269–73.
Stokes TA, Holston A, Olsen C, Choi Y, Curtis J, Higginson J, et al. Preterm infants of lower gestational age at birth have greater waist circumference-length ratio and ponderal index at term age than preterm infants of higher gestational ages. J Pediatr. 2012;161:735–41.
Johnson MJ, Wootton SA, Leaf AA, Jackson AA. Preterm birth and body composition at term equivalent age: a systematic review and meta-analysis. Pediatrics. 2012;130:e640–e649.
Ramel SE, Zhang L, Misra S, Anderson CG, Demerath EW. Do anthropometric measures accurately reflect body composition in preterm infants? Pedia Obes. 2017;12(Suppl 1):72–77.
Perng W, Ringham BM, Glueck DH, Sauder KA, Starling AP, Belfort MB, et al. An observational cohort study of weight- and length-derived anthropometric indicators with body composition at birth and 5 mo: the Healthy Start study. Am J Clin Nutr. 2017;106:559–67.
Roy SM, Spivack JG, Faith MS, Chesi A, Mitchell JA, Kelly A, et al. Infant BMI or weight-for-length and obesity risk in early childhood. Pediatrics. 2016;137:2015–3492.
López-Jaramillo P, Gómez-Arbeláez D, López-López J, López-López C, Martínez-Ortega J, Gómez-Rodríguez A, et al. The role of leptin/adiponectin ratio in metabolic syndrome and diabetes. Horm Mol Biol Clin Investig. 2014;18:37–45.
Nakano Y, Itabashi K, Sakurai M, Aizawa M, Dobashi K, Mizuno K. Accumulation of subcutaneous fat, but not visceral fat, is a predictor of adiponectin levels in preterm infants at term-equivalent age. Early Hum Dev. 2014;90:213–7.
Ehrenkranz RA, Dusick AM, Vohr BR, Wright LL, Wrage LA, Poole WK. Growth in the neonatal intensive care unit influences neurodevelopmental and growth outcomes of extremely low birth weight infants. Pediatrics. 2006;117:1253–61.
Belfort MB, Rifas-Shiman SL, Sullivan T, Collins CT, McPhee AJ, Ryan P, et al. Infant growth before and after term: effect on neurodevelopment in preterm infants. Pediatrics. 2011;128:e899–906.
Villar J, Giulani F, Barros F, Roggero P, Coronado Zarco IA, MAS Rego, et al. Monitoring the postnatal growth of preterm infants: a paradigm change. Pediatrics. 2018;141:e20172467.
Rozé JC, Darmaun D, Boquien CY, Flamant C, Picaud JC, Savagner C, et al. The apparent breastfeeding paradox in very preterm infants: relationship between breast feeding, early weight gain and neurodevelopment based on results from two cohorts, EPIPAGE and LIFT. BMJ Open. 2012;2:e000834.
Meyers JM, Tan S, Bell EF, Duncan AF, Guillet R, Stoll BJ, et al. Neurodevelopmental outcomes among extremely premature infants with linear growth restriction. J Perinatol. 2019;39:193–202.
C. W Levenson, Morris D. Zinc and neurogenesis: making new neurons from development to adulthood. Adv Nutr 2011;2:96–100.
Acknowledgements
Lara Pavageau, MD, validated and implemented serial length measurements that allowed characterization of growth patterns; these data were published previously [18]. Susan Chacko, RN and Maria DeLeon, RN were research coordinators for part of this study. Chen Du, RD, Elizabeth Brammer, RD, Audrey Edwards, RD, and Theresa Jacob, RD, dietitians at Parkland Hospital, obtained anthropometric measurements for this study and participated in patient recruitment, assessments of growth and laboratory results and recommendations for nutritional interventions. Elen Petrosyan, RD, dietitian at Parkland Hospital, helped organizing the logistics of patient recruitment, measurements and research planning. Christopher Clark, IT specialist at Parkland Hospital, extracted data from EPIC at Parkland. Sandra Gosser, NNP, helped with education of nurse practitioners and coordination of care with the dietitians and the physicians. Timothy Brannon, MD, helped with changes on EPIC, specifically with implementation of parameters to define AGA at birth. Rebecca Thomas, RN and Catherine Vanbeek, RN, NP, helped with changes in EPIC ordering and education of nurses. Lizette Torres, RN, Alicia Guzman, Cathy Boatman, Emily Lentz, RD, and Elizabeth Heyne, PA-C, helped with collection of follow-up data. Some of the patients included in the QI described in the current manuscript were enrolled in a blinded randomized trial: Clinicaltrials.gov NCT02372136. Preliminary results were submitted in abstract form to the following meetings: Brion LP, Rosenfeld CR, Heyne R, Brown LS, Lair C, Burchfield P. Quality Improvement Project Designed to Decrease Weight-Length Disproportion at Discharge in Very Preterm Infants. Poster presentation at AAP NCE, Orlando, FL, 11/2/2018. Brion LP, Rosenfeld CR, Heyne R, Brown LS, Lair C, Burchfield P, Caraig M. Adjustable Feedings plus Accurate Serial Length Measurements Can Decrease Weight-Length Disproportion in Very Preterm Infants. Poster presentation at Pediatric Academy Societies, Baltimore, MD, 4/29/2019.
Financial Support
George L. MacGregor Professorship (CRR) and Children’s Medical Center Clinical Advisory Committee (CCRAC) – Senior Investigator Research Award – New Direction (LPB, 2015–17)
Author information
Authors and Affiliations
Contributions
LPB: wrote the first draft of the manuscript. He conceptualized and designed the study. He collected and reviewed data from the database, participated in the interpretation of the data, conducted statistical analyses, critically reviewed the revisions, and approved the final manuscript as submitted. CRR: conceptualized and designed the study. He participated in the interpretation of the data, critically reviewed the revisions, and approved the final manuscript as submitted. RH: conceptualized and designed the study. He participated in the interpretation of the data, critically reviewed the revisions, and approved the final manuscript as submitted. LSB: conceptualized and designed the study. He calculated processes of care using data extracted electronically from EPIC, conducted statistical analyses, participated in the interpretation of the data, critically reviewed the revisions, and approved the final manuscript as submitted. CSL: conceptualized and designed nutritional guidelines for the NICU and discharge, anthropometric data and data extraction from EPIC. She obtained anthropometric measurements for this study and participated in patient recruitment, assessments of growth and laboratory results and recommendations for nutritional interventions. She participated in the interpretation of the data, critically reviewed the revisions, and approved the final manuscript as submitted. PJB: collected and entered data into the databases; she participated in the interpretation of the data, critically reviewed the manuscript and approved the final manuscript as submitted. MC: was research coordinator for a large part of this study. She recruited and measured patients in the NICU and at follow-up. She extracted data from medical records into databases, critically reviewed the manuscript and approved the final manuscript as submitted.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Brion, L.P., Rosenfeld, C.R., Heyne, R. et al. Adjustable feedings plus accurate serial length measurements decrease discharge weight-length disproportion in very preterm infants: quality improvement project. J Perinatol 39, 1131–1139 (2019). https://doi.org/10.1038/s41372-019-0424-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41372-019-0424-8
This article is cited by
-
Follow-up of a randomized trial optimizing neonatal nutrition in preterm very low birthweight infants: growth, serum adipokines, renal function and blood pressure
Journal of Perinatology (2024)
-
Growth after implementing a donor breast milk program in neonates <33 weeks gestational age or birthweight <1500 grams: Retrospective cohort study
Journal of Perinatology (2023)
-
Double-blinded randomized controlled trial of optimizing nutrition in preterm very low birth weight infants: Bayley scores at 18–38 months of age
Journal of Perinatology (2023)
-
Hyperglycemia and prematurity: a narrative review
Pediatric Research (2023)
-
Quality improvement project designed to reduce disproportionate growth in extremely low gestational age neonates: cognitive neurodevelopmental outcome at 18–41 months
Journal of Perinatology (2021)