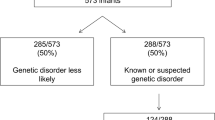
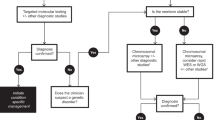
Abstract
Infants who die within the first weeks to months of life may have genetic disorders, though many die without a confirmed diagnosis. Non-genetic conditions may also be responsible for unexplained infant deaths, and the diagnosis may be reliant upon studies performed in the peri-mortem period. Neonatologists, obstetricians, or pediatricians caring for these children and their families may be unsure of which investigations can and should be performed in the setting of a newborn or infant who is dying or has died. Recent advances in genomic sequencing technology may provide additional diagnostic options, though the interpretation of genetic variants discovered by this technique may be contingent upon clinical phenotype information that is obtained peri-mortem or upon autopsy. We have reviewed the current literature concerning the evaluation of an unexplained neonatal or infantile demise and synthesized a diagnostic approach, with a focus on the contribution of new and emerging genomic technologies.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Sankaran K, Chien LY, Walker R, Seshia M, Ohlsson A, Canadian Neonatal N. Variations in mortality rates among Canadian neonatal intensive care units. CMAJ. 2002;166:173–8.
Simpson CD, Ye XY, Hellmann J, Tomlinson C. Trends in cause-specific mortality at a Canadian outborn NICU. Pediatrics. 2010;126:1538.
Hudome SM, Kirby RS, Senner JW, Cunniff C. Contribution of genetic disorders to neonatal mortality in a regional intensive care setting. Am J Perinatol. 1994;11:100–3.
Jacob J, Kamitsuka M, Clark RH, Kelleher AS, Spitzer AR. Etiologies of NICU deaths. Pediatrics. 2015;135:59.
Weiner J, Sharma J, Lantos J, Kilbride H. How infants die in the neonatal intensive care unit: trends from 1999 through 2008. Arch Pediatr Adolesc Med. 2011;165:630–4.
Wojcik MH, Schwartz T, Yamin I, Edward H, Genetti C, Towne M, et al. Genetic Disorders and Mortality in Infancy and Early Childhood: Delayed Diagnoses and Missed Opportunities. Genet Med https://doi.org/10.1038/gim.2018.17.
Morris JA. The genomic load of deleterious mutations: relevance to death in infancy and childhood. Front Immunol. 2015;6:105.
Weber MA, Ashworth MT, Risdon RA, Hartley JC, Malone M, Sebire NJ. The role of postmortem investigations in determining the cause of sudden unexpected death in infancy. Arch Dis Child. 2008;93:1048–53.
Goldstein RD, Kinney HC, Willinger M. Sudden unexpected death in fetal life through early childhood. Pediatrics. 2016;137:4661.
Mathews TJ, Driscoll AK. Trends in Infant Mortality in the United States, 2005–2014. NCHS data brief, no. 279. Hyattsville, MD: National Center for Health Statistics; 2017.
Michel MC, Colaizy TT, Klein JM, Segar JL, Bell EF. Causes and circumstances of death in a neonatal unit over 20 years. Pediatr Res. 2018;83:829–33.
Costa S, Rodrigues M, Centeno MJ, Martins A, Vilan A, Brandao O, et al. Diagnosis and cause of death in a neonatal intensive care unit--how important is autopsy? J Matern Fetal Neonatal Med. 2011;24:760–3.
Weber MA, Ashworth MT, Risdon RA, Brooke I, Malone M, Sebire NJ. Sudden unexpected neonatal death in the first week of life: autopsy findings from a specialist centre. J Matern Fetal Neonatal Med. 2009;22:398–404.
Baruteau AE, Tester DJ, Kapplinger JD, Ackerman MJ, Behr ER. Sudden infant death syndrome and inherited cardiac conditions. Nat Rev Cardiol. 2017;14:715–26.
Shamseldin HE, Kurdi W, Almusafri F, Alnemer M, Alkaff A, Babay Z, et al. Molecular autopsy in maternal-fetal medicine. Genet Med. 2018;20:420–7.
Meng L, Pammi M, Saronwala A, Magoulas P, Ghazi AR, Vetrini F, et al. Use of exome sequencing for infants in intensive care units: ascertainment of severe single-gene disorders and effect on medical management. JAMA Pediatr. 2017;171:e173438.
Saunders CJ, Miller NA, Soden SE, Dinwiddie DL, Noll A, Alnadi NA, et al. Rapid whole-genome sequencing for genetic disease diagnosis in neonatal intensive care units. Sci Transl Med. 2012;4:154ra35.
Middleton O, Baxter S, Demo E, Honeywell C, Jentzen J, Miller F, et al. National association of medical examiners position paper: retaining postmortem samples for genetic testing. Acad Forensic Pathol. 2013;3:191–4.
Wright C, Lee RE. Investigating perinatal death: a review of the options when autopsy consent is refused. Arch Dis Child Fetal Neonatal Ed. 2004;89:285.
British Association for Perinatal Medicine. Guidelines for the investigation of newborn infants who suffer a sudden and unexpected postnatal collapse in the first week of life: recommendations from a professional group on sudden unexpected postnatal collapse. London. 2011.
McPherson E, Nestoridi E, Heinke D, Roberts DJ, Fretts R, Yazdy MM, et al. Alternatives to autopsy for fetal and early neonatal (Perinatal) deaths: insights from the Wisconsin Stillbirth Service Program. Birth Defects Res. 2017;109:1430–41.
Iyasu S, Rowley DL, Hanzlick RL. Guidelines for death scene investigation of sudden, unexplained infant deaths: recommendations of the interagency panel on sudden infant death syndrome. Morb Mortal Wkly Rep. 1996;45:22.
Cummings BB, Marshall JL, Tukiainen T, Lek M, Donkervoort S, Foley AR, et al. Improving genetic diagnosis in Mendelian disease with transcriptome sequencing. Sci Transl Med. 2017;9: pii: eaal5209.
Carithers LJ, Ardlie K, Barcus M, Branton PA, Britton A, Buia SA, et al. A novel approach to high-quality postmortem tissue procurement: the GTEx project. Biopreserv Biobank. 2015;13:311–9.
Griscom NT, Driscoll SG. Radiography of stillborn fetuses and infants dying at birth. Am J Roentgenol. 1980;134:485–9.
Thayyil S, Sebire NJ, Chitty LS, Wade A, Chong W, Olsen O, et al. Postmortem MRI versus conventional autopsy in fetuses and children: a prospective validation study. Lancet. 2013;382:223–33.
Leadbetter KZ, Vesoulis ZA, White FV, Schmidt RE, Khanna G, Shimony JS, et al. The role of postmortem MRI in the neonatal intensive care unit. J Perinatol. 2017;37:98–103.
Nijkamp JW, Sebire NJ, Bouman K, Korteweg FJ, Erwich JJHM, Gordijn SJ. Perinatal death investigations: what is current practice? Semin Fetal Neonatal Med. 2017;22:167–75.
Nese N, Bulbul Y. Diagnostic value of perinatal autopsies: analysis of 486 cases. J Perinat Med. 2018;46:175–81.
Yamamoto T, Mishima H, Mizukami H, Fukahori Y, Umehara T, Murase T, et al. Metabolic autopsy with next generation sequencing in sudden unexpected death in infancy: postmortem diagnosis of fatty acid oxidation disorders. Mol Genet Metab Rep. 2015;5:26–32.
Miller DT, Adam MP, Aradhya S, Biesecker LG, Brothman AR, Carter NP, et al. Consensus statement: chromosomal microarray is a first-tier clinical diagnostic test for individuals with developmental disabilities or congenital anomalies. Am J Hum Genet. 2010;86:749–64.
Wapner RJ, Martin CL, Levy B, Ballif BC, Eng CM, Zachary JM, et al. Chromosomal microarray versus karyotyping for prenatal diagnosis. N Engl J Med. 2012;367:2175–84.
Reddy UM, Page GP, Saade GR, Silver RM, Thorsten VR, Parker CB, et al. Karyotype versus microarray testing for genetic abnormalities after stillbirth. N Engl J Med. 2012;367:2185–93.
Talkowski ME, Ordulu Z, Pillalamarri V, Benson CB, Blumenthal I, Connolly S, et al. Clinical diagnosis by whole-genome sequencing of a prenatal sample. N Engl J Med. 2012;367:2226–32.
Grody WW, Thompson BH, Hudgins L. Whole-exome/genome sequencing and genomics. Pediatrics. 2013;132(Suppl 3):211.
Ewans LJ, Schofield D, Shrestha R, Zhu Y, Gayevskiy V, Ying K, et al. Whole-exome sequencing reanalysis at 12 months boosts diagnosis and is cost-effective when applied early in Mendelian disorders. Genet Med https://doi.org/10.1038/gim.2018.39.
Hutchinson JC, Arthurs OJ, Sebire NJ. Postmortem research: innovations and future directions for the perinatal and paediatric autopsy. Arch Dis Child Educ Pract Ed. 2016;101:54–6.
Filges I, Friedman JM. Exome sequencing for gene discovery in lethal fetal disorders--harnessing the value of extreme phenotypes. Prenat Diagn. 2015;35:1005–9.
Oshima Y, Yamamoto T, Ishikawa T, Mishima H, Matsusue A, Umehara T, et al. Postmortem genetic analysis of sudden unexpected death in infancy: neonatal genetic screening may enable the prevention of sudden infant death. J Hum Genet. 2017;62:989–95.
Yang Y, Muzny DM, Reid JG, Bainbridge MN, Willis A, Ward PA, et al. Clinical whole-exome sequencing for the diagnosis of mendelian disorders. N Engl J Med. 2013;369:1502–11.
Soden SE, Saunders CJ, Willig LK, Farrow EG, Smith LD, Petrikin JE, et al. Effectiveness of exome and genome sequencing guided by acuity of illness for diagnosis of neurodevelopmental disorders. Sci Transl Med. 2014;6:265ra168.
Willig LK, Petrikin JE, Smith LD, Saunders CJ, Thiffault I, Miller NA, et al. Whole-genome sequencing for identification of Mendelian disorders in critically ill infants: a retrospective analysis of diagnostic and clinical findings. Lancet Respir Med. 2015;3:377–87.
Smith LD, Willig LK, Kingsmore SF. Whole-exome sequencing and whole-genome sequencing in critically ill neonates suspected to have single-gene disorders. Cold Spring Harb Perspect Med. 2015;6:a023168.
Stark Z, Tan TY, Chong B, Brett GR, Yap P, Walsh M, et al. A prospective evaluation of whole-exome sequencing as a first-tier molecular test in infants with suspected monogenic disorders. Genet Med. 2016;18:1090–6.
Tan TY, Dillon OJ, Stark Z, Schofield D, Alam K, Shrestha R, et al. Diagnostic impact and cost-effectiveness of whole-exome sequencing for ambulant children with suspected monogenic conditions. JAMA Pediatr. 2017;171:855–62.
Richards S, Aziz N, Bale S, Bick D, Das S, Gastier-Foster J, et al. Standards and guidelines for the interpretation of sequence variants: a joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet Med. 2015;17:405–24.
National Society of Genetic Counselors. Postmortem Genetic Testing FAQs. https://www.nsgc.org/postmortem. Updated 2017. Accessed 2017.
Acknowledgements
We thank Drs. Christine Bryke and Jonathan Hecht for their assistance in the development of an earlier version of our clinical algorithm for use at Beth Israel Deaconess Medical Center. MH Wojcik is supported by NIH T32GM007748.
Funding:
NIH T32GM007748 [MHW].
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Wojcik, M.H., Brodsky, D., Stewart, J.E. et al. Peri-mortem evaluation of infants who die without a diagnosis: focus on advances in genomic technology. J Perinatol 38, 1125–1134 (2018). https://doi.org/10.1038/s41372-018-0187-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41372-018-0187-7
This article is cited by
-
Multidisciplinary Workup for Stillbirth at a Tertiary-Care Hospital in Northeast Mexico: Findings, Challenges and Perspectives
Maternal and Child Health Journal (2024)
-
The Mortality of Periviable and Extremely Premature Infants and Their Impact on the Overall Neonatal Mortality Rate
Scientific Reports (2020)
-
Infant mortality: the contribution of genetic disorders
Journal of Perinatology (2019)