Abstract
Objective
To characterize neonatal iron stores depending on gestational age (GA) at term.
Study design
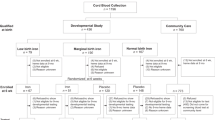
Participants were 751 mother–newborn pairs from the placebo arm of a randomized clinical trial of prenatal iron-folate supplementation in China. We compared mean cord serum ferritin (SF) by weeks GA and, following the general linear model, assessed whether maternal iron deficiency (ID) influenced relations between GA and cord SF.
Results
Controlling for covariates, cord SF increased between 37 and 41 weeks (ps < 0.05–0.01). Cord SF was lower in infants of ID vs. non-ID mothers (geometric mean 96.3 [95% CI: 91.3–101.6] µg/L vs. 115.9 [95% CI: 105.0–127.8] µg/L, effect size = 0.33 SD, p = 0.0012). There was no significant increase with GA among infants of ID mothers. For non-ID mothers, cord-blood SF increased sharply with GA until 38 5/7 weeks, after which it plateaued.
Conclusions
The findings emphasize that neonates at 37–38 weeks, although considered term, are not fully mature.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Domelloff M, Georgieff MK. Post-discharge iron requirements of the preterm infant. J Pediatr. 2015;167:S31–5.
Spong CY. Defining “term” pregnancy: recommendations from the defining “term” pregnancy workgroup. JAMA. 2013;309:2445–6.
American College of Obstetricians and Gynecologists. Definition of term pregnancy. Committee Opinion No. 579. Obstet Gynecol. 2013;122:1139–40.
Lorenz L, Peter A, Poets CF, Franz AR. A review of cord blood concentrations of iron status parameters to define reference ranges for preterm infants. Neonatology . 2013;104:194–202.
Siddappa AM, Rao R, Long JD, Widness JA, Georgieff MK. The assessment of newborn iron stores at birth: a review of the literature and standards for ferritin concentrations. Neonatology. 2007;92:73–82.
WHO. Guideline: Daily iron and folic acid supplementation in pregnant women. Geneva: World Health Organization; 2012.
Stevens GA, Finucane MM, De-Regil LM, Paciorek CJ, Flaxman SR, Branca F, Pena-Rosas JP, Bhutta ZA, Ezzati M. Global, regional, and national trends in haemoglobin concentration and prevalence of total and severe anaemia in children and pregnant and non-pregnant women for 1995–2011: a systematic analysis of population-representative data. Lancet Glob Health. 2013;1:e16–e25.
Zhao G, Xu G, Zhou M, Jiang Y, Richards B, Clark KM, Kaciroti N, Georgieff MK, Zhang Z, Tardif T, Li M, Lozoff B. Prenatal iron supplementation reduces maternal anemia, iron deficiency, and iron deficiency anemia in a randomized clinical trial in rural China, but iron deficiency remains widespread in mothers and neonates. J Nutr. 2015;145:1916–23.
WHO. Serum ferritin concentrations for the assessment of iron status and iron deficiency in populations. Vitamin and Mineral Nutrition Information System. Geneva: World Health Organization; 2011. http://www.who.int/vmnis/indicators/ferritin/en/index.html. Accessed 14 July 2014.
McGee VE. Piecewise regression. J Am Stat Assoc. 1970;65:1109–24.
Choi JW, Kim CS, Pai SH. Erythropoietic activity and soluble transferrin receptor level in neonates and maternal blood. Acta Paediatr. 2000;89:675–9.
Hay G, Refsum H, Whitelaw A, Melbye EL, Haug E, Borch-Iohnsen B. Predictors of serum ferritin and serum soluble transferrin receptor in newborns and their associations with iron status during the first 2 y of life. Am J Clin Nutr. 2007;86:64–73.
Shao J, Lou J, Rao R, Georgieff MK, Kaciroti N, Felt BT, Zhao ZY, Lozoff B. Maternal serum ferritin concentration is positively associated with newborn iron stores in women with low ferritin status in late pregnancy. J Nutr. 2012;142:2004–9.
Rao R, Georgieff MK. Iron in fetal and neonatal nutrition. Semin Fetal Neonatal Med. 2007;12:54–63.
Vricella LK. Emerging understanding and measurement of plasma volume expansion in pregnancy. Am J Clin Nutr. 2017;106:1620S–5S.
Dewey KG, Oaks BM. U-shaped curve for risk associated with maternal hemoglobin, iron status, or iron supplementation. Am J Clin Nutr. 2017;106:1694S–1702S.
Acknowledgements
This analysis was based on a RCT supported by Vifor Pharma Limited (Switzerland). Measures of maternal and newborn iron status were supported by a grant from US National Institutes of Health (R01 HD052069), which included funding from the Eunice Kennedy Shriver National Institute of Child Health and Human Development and the Office of Dietary Supplements. Iron supplements and placebo were prepared by Lee’s Pharmaceutical Holdings Limited (Hong Kong) and provided at cost. We thank Drs. Gengli Zhao and Min Zhou for overall direction of the pregnancy RCT, Drs. Zhixiang Zhang and Twila Tardif for their leadership roles in coordinating and supervising the project, Dr. Xintian Lu for input about iron status measures and supervision of Ying Hua, Dr. Michael Georgieff for critical comments and constructive suggestions on the manuscript, and Ms. Katy Clark for help preparing the manuscript. We also appreciate the dedicated efforts of project doctors and nurses at Sanhe Maternal and Child Health Center.
Funding
This analysis was based on a randomized clinical trial supported by Vifor Pharma Limited (Switzerland). Measures of maternal and newborn iron status were supported by a grant from US National Institutes of Health (R01 HD052069), which included funding from the Eunice Kennedy Shriver National Institute of Child Health and Human Development and the Office of Dietary Supplements. The study sponsors had no role in the study design, collection, analysis, and interpretation of data, writing of the report, or decision to submit for publication. The content is solely the responsibility of the authors and does not necessarily represent the official views of the funding agencies. Iron supplements and placebo were prepared by Lee’s Pharmaceutical Holdings Limited (Hong Kong) and provided at cost. This clinical trial was registered at ClinicalTrials.gov, NCT02221752.
Author contribution
YH conceived the research question, performed initial data analysis, wrote the first draft, and approved the final version. NK conceived the analytic approach, performed final data analysis, revised the manuscript, and approved the final version. YJ established and performed laboratory iron studies, participated in conceiving the study, revised the manuscript, and approved the final version. XL assisted in organizing the pregnancy study, including cord blood iron collection, participated in conceiving the research question, revised the manuscript, and approved the final version. GX designed and supervised laboratory iron studies, participated in conceiving the analysis, revised the manuscript, and approved the final version. BR participated in conceiving the research question, performed data analysis, revised the manuscript, and approved the final version. ML helped conceptualize the pregnancy trial, organized and supervised the pregnancy and infant components, critically reviewed the manuscript, and approved the final version. BL conceptualized the pregnancy trial, organized and supervised the pregnancy and infant components, critically reviewed and revised the manuscript, and approved the final version.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Hua, Y., Kaciroti, N., Jiang, Y. et al. Inadequate iron stores in early term neonates. J Perinatol 38, 1017–1021 (2018). https://doi.org/10.1038/s41372-018-0140-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41372-018-0140-9