Abstract
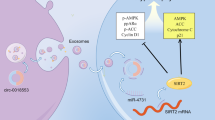
Through previous literature studies, we found that miR-124-3p may be associated with hypertension. Therefore, we investigated the relationship between miR-124-3p and hypertension in Human Umbilical Vein Endothelial Cells (HUVECs) induced by angiotensin II (AngII). AngII-induced HUVECs model was constructed and the expression of miR-124-3p was detected by qRT-PCR. After transfected cells, apoptosis and ROS production were detected by flow cytometry, caspase-3 kit, and DCFH-DA staining. The target genes of miR-124-3p were predicted and verified by TargrtScan, Luciferase assay, qRT-PCR, and western blot. After silencing Early growth response factor 1 (siEGR1), its effects on apoptosis and ROS production were explored. Finally, the rescue experiments were conducted to explore the mechanism of miR-124-3p to reduce hypertension. MiR-124-3p was underexpressed in the cell model. In Ang II-induced HUVECs, the number of apoptosis increased, the content of caspase-3 was higher, and ROS production increased. However, these effects could be partially inhibited by miR-124-3p mimic. EGR1 was down-regulated by miR-124-3p, and siEGR1 was able to inhibit apoptosis and ROS production of cell model. In the final rescue experiments, miR-124-3p partially reversed the effect of Ang-II on the viability, migration, invasion and apoptosis and ROS production in HUVECs by down-regulating EGR1. MiR-124-3p inhibits Ang II-induced apoptosis and ROS production in HUVECs by down-regulating EGR1.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
References
Lawes CM, Vander Hoorn S, Rodgers A. Global burden of blood-pressure-related disease, 2001. Lancet (Lond, Engl). 2008;371:1513–8.
Wang Z, Chen Z, Zhang L, Wang X, Hao G, Zhang Z, et al. Status of hypertension in china: results from the china hypertension survey, 2012-2015. Circulation. 2018;137:2344–56.
Doyle AE. Hypertension and vascular disease. Am J hypertension. 1991;4:103s–106s.
Cuspidi C, Tadic M, Grassi G, Mancia G. Treatment of hypertension: the ESH/ESC guidelines recommendations. Pharmacol Res. 2018;128:315–21.
Vaziri ND, Rodriguez-Iturbe B. Mechanisms of disease: oxidative stress and inflammation in the pathogenesis of hypertension. Nat Clin Pract Nephrol. 2006;2:582–93.
van Thiel BS, van der Pluijm I, te Riet L, Essers J, Danser AH. The renin-angiotensin system and its involvement in vascular disease. Eur J Pharmacol. 2015;763:3–14.
Miyazaki M. [Angiotensin II in organ organopathy]. Nihon Rinsho. 2004;62:21–7.
Mirabito Colafella KM, Danser AHJ. Recent advances in angiotensin research. Hypertension (Dallas, Tex: 1979). 2017;69:994–99.
Rajagopalan S, Harrison DG. Reversing endothelial dysfunction with ACE inhibitors. A new trend. Circulation. 1996;94:240–3.
Oliveras A, de la Sierra A. Resistant hypertension: patient characteristics, risk factors, co-morbidities and outcomes. J Hum Hypertension. 2014;28:213–7.
Rupaimoole R, Slack FJ. MicroRNA therapeutics: towards a new era for the management of cancer and other diseases. Nat Rev Drug Discov. 2017;16:203–22.
Backes C, Meese E, Keller A. Specific miRNA disease biomarkers in blood, serum and plasma: challenges and prospects. Mol Diagn Ther. 2016;20:509–18.
Wang Y, Chen L, Wu Z, Wang M, Jin F, Wang N, et al. miR-124-3p functions as a tumor suppressor in breast cancer by targeting CBL. BMC Cancer. 2016;16:826.
Idichi T, Seki N, Kurahara H, Fukuhisa H, Toda H, Shimonosono M, et al. Involvement of anti-tumor miR-124-3p and its targets in the pathogenesis of pancreatic ductal adenocarcinoma: direct regulation of ITGA3 and ITGB1 by miR-124-3p. Oncotarget. 2018;9:28849–65.
de Ronde MWJ, Kok MGM, Moerland PD, Van den Bossche J, Neele AE, Halliani A, et al. High miR-124-3p expression identifies smoking individuals susceptible to atherosclerosis. Atherosclerosis. 2017;263:377–84.
Rainer TH, Leung LY, Chan CPY, Leung YK, Abrigo JM, Wang D, et al. Plasma miR-124-3p and miR-16 concentrations as prognostic markers in acute stroke. Clin Biochem. 2016;49:663–68.
Chen W, Yu F, Di M, Li M, Chen Y, Zhang Y, et al. MicroRNA-124-3p inhibits collagen synthesis in atherosclerotic plaques by targeting prolyl 4-hydroxylase subunit alpha-1 (P4HA1) in vascular smooth muscle cells. Atherosclerosis. 2018;277:98–107.
Chen DG, Zhu B, Lv SQ, Zhu H, Tang J, Huang C, et al. Inhibition of EGR1 inhibits glioma proliferation by targeting CCND1 promoter. J Exp Clin Cancer Res. 2017;36:186.
Khachigian LM. Early growth response-1 in the pathogenesis of cardiovascular disease. J Mol Med (Berl, Ger). 2016;94:747–53.
Gaut L, Robert N, Delalande A, Bonnin MA, Pichon C, Duprez D. EGR1 regulates transcription downstream of mechanical signals during tendon formation and healing. PloS ONE. 2016;11:e0166237.
Shin SY, Lee JM, Lim Y, Lee YH. Transcriptional regulation of the growth-regulated oncogene alpha gene by early growth response protein-1 in response to tumor necrosis factor alpha stimulation. Biochimica et Biophysica Acta 2013; 1829: 1066–74.
Han MH, Park C, Jin CY, Kim GY, Chang YC, Moon SK, et al. Apoptosis induction of human bladder cancer cells by sanguinarine through reactive oxygen species-mediated up-regulation of early growth response gene-1. PloS ONE. 2013;8:e63425.
Bhindi R, Khachigian LM, Lowe HC. DNAzymes targeting the transcription factor Egr-1 reduce myocardial infarct size following ischemia-reperfusion in rats. J Thrombosis Haemost. 2006;4:1479–83.
Kang JM, Kim N, Kim JH, Oh E, Lee BY, Lee BH, et al. Effect of aging on gastric mucosal defense mechanisms: ROS, apoptosis, angiogenesis, and sensory neurons. Am J Physiol Gastrointest Liver Physiol. 2010;299:G1147–53.
Elmore S. Apoptosis: a review of programmed cell death. Toxicol Pathol. 2007;35:495–516.
Charununtakorn ST, Shinlapawittayatorn K, Chattipakorn SC, Chattipakorn N. Potential roles of humanin on apoptosis in the heart. Cardiovasc Ther. 2016;34:107–14.
Sinha N, Dabla PK. Oxidative stress and antioxidants in hypertension-a current review. Curr Hypertens Rev. 2015;11:132–42.
Vaziri ND, Wang XQ, Oveisi F, Rad B. Induction of oxidative stress by glutathione depletion causes severe hypertension in normal rats. Hypertension. 2000;36:142–6.
Wang D, Strandgaard S, Iversen J, Wilcox CS. Asymmetric dimethylarginine, oxidative stress, and vascular nitric oxide synthase in essential hypertension. Am J Physiol Regul Integr Comp Physiol. 2009;296:R195–200.
Sturtzel C. Endothelial cells. Adv Exp Med Biol. 2017;1003:71–91.
Barbacena P, Carvalho JR, Franco CA. Endothelial cell dynamics in vascular remodelling. Clin Hemorheol Microcirc. 2016;64:557–563.
Versari D, Daghini E, Virdis A, Ghiadoni L, Taddei S. Endothelium-dependent contractions and endothelial dysfunction in human hypertension. Br J Pharmacol. 2009;157:527–36.
Konukoglu D, Uzun H. Endothelial dysfunction and hypertension. Adv Exp Med Biol. 2017;956:511–40.
Funding
This work was supported by the Lishui public welfare Technology Application Research Project [2019GYX28]; the Zhejiang basic public welfare research plan [LGF19H02008].
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Lv, L., Shen, J., Xu, J. et al. MiR-124-3p reduces angiotensin II-dependent hypertension by down-regulating EGR1. J Hum Hypertens 35, 696–708 (2021). https://doi.org/10.1038/s41371-020-0381-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41371-020-0381-x
This article is cited by
-
Ferroptosis-related genes, a novel therapeutic target for focal segmental glomerulosclerosis
BMC Nephrology (2024)
-
The pharmaco-epigenetics of hypertension: a focus on microRNA
Molecular and Cellular Biochemistry (2024)
-
Identifying the Interaction Between Tuberculosis and SARS-CoV-2 Infections via Bioinformatics Analysis and Machine Learning
Biochemical Genetics (2023)
-
Ibandronate promotes autophagy by inhibiting Rac1–mTOR signaling pathway in vitro and in vivo
Cell Death Discovery (2022)
-
MiR-124 and miR-506 are involved in the decline of protein C in children with extra-hepatic portal vein obstruction
Scientific Reports (2021)