Abstract
Background
Organophosphate esters (OPEs) are ubiquitously detected in environments and their exposure may affect respiratory health. However, epidemiological evidence, particularly among adolescents, is very limited.
Objective
We aimed to investigate the associations of urinary OPEs metabolites with asthma and lung function among adolescents and to identify potential effect modifiers.
Methods
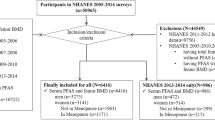
Included were 715 adolescents aged 12–19 years old participating in the National Health and Nutrition Examination Survey (NHANES) 2011–2014. Multivariable binary logistic regression and linear regression were used to assess associations with asthma and lung function, respectively. Stratified analyses were conducted to assess the effect modifications of serum sex hormones, vitamin D levels, and body mass index (BMI).
Results
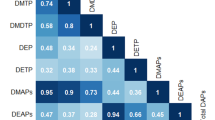
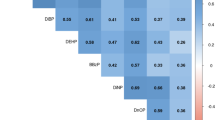
After multivariable adjustment, we found that bis(2-chloroethyl) phosphate (BCEP) (3rd tertile [T3] vs 1st tertile [T1], OR = 1.87, 95% CI: 1.08, 3.25; P-trend=0.029) and diphenyl phosphate (DPHP) (T3 vs T1, OR = 2.52, 95%CI: 1.25, 5.04; P-trend=0.013) were associated with elevated odds of asthma in all adolescents. Sex-stratified analyses revealed that associations of these two OPEs metabolites tended to be stronger in males. Meanwhile, BCEP and the molecular sum of OPEs metabolites (∑OPEs) were significantly associated with declined lung function, either in all adolescents or by sex. Furthermore, stratified analyses revealed that positive associations of OPEs metabolites with asthma tended to be stronger among adolescents with insufficient levels of Vitamin D (VD < 50 nmol/L), relatively high levels of total testosterone (≥356 ng/dL and ≥22.5 ng/dL for males and females, respectively), or low levels of estradiol (<19.1 pg/mL and <47.3 pg/mL for males and females, respectively).
Significance
Certain urinary OPEs metabolites, especially DPHP and BCEP, were associated with elevated odds of asthma and declined lung function in adolescents. Such associations might be partly modified by levels of VD and sex steroid hormones.
Impact statement
The observed associations of urinary OPEs metabolites with increased risk of asthma and declined lung function highlight the potential hazard of OPEs exposure to respiratory health among adolescents.

This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 6 print issues and online access
$259.00 per year
only $43.17 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author upon reasonable request.
References
Yonemoto N. Global burden of 369 diseases and injuries in 204 countries and territories, 1990-2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet. 2020;396:1204–22.
Mims JW. Asthma: definitions and pathophysiology. Int forum allergy Rhinol. 2015;5(Suppl 1):S2–6.
CDC. Most recent national asthma data. National current asthma prevalence (2019). 2021. https://www.cdc.gov/asthma/most_recent_national_asthma_data.htm.
Pate CA, Zahran HS, Qin X, Johnson C, Hummelman E, Malilay J. Asthma surveillance - United States, 2006-2018. MMWR Surveill Summ. 2021;70:1–32.
Mallol J, Aguirre V, Mallol-Simmonds M, Matamala-Bezmalinovic A, Calderón-Rodriguez L, Osses-Vergara F. Changes in the prevalence of asthma and related risk factors in adolescents: three surveys between 1994 and 2015. Allergol Immunopathol. 2019;47:313–21.
Guarnieri M, Balmes JR. Outdoor air pollution and asthma. Lancet. 2014;383:1581–92.
Murrison LB, Brandt EB, Myers JB, Hershey GKK. Environmental exposures and mechanisms in allergy and asthma development. J Clin Invest. 2019;129:1504–15.
Percy Z, Vuong AM, Xu Y, Xie C, Ospina M, Calafat AM, et al. Maternal urinary organophosphate esters and alterations in maternal and neonatal thyroid hormones. Am J Epidemiol. 2021;190:1793–802.
Luo K, Liu J, Wang Y, Aimuzi R, Luo F, Ao J, et al. Associations between organophosphate esters and sex hormones among 6-19-year old children and adolescents in NHANES 2013-4. Environ Int. 2020;136:105461.
Luo K, Aimuzi R, Wang Y, Min N, Zhang J. Urinary organophosphate esters metabolites, glucose homeostasis and prediabetes in adolescents. Environ Pollut. 2020;267:115607.
Luo K, Zhang R, Aimuzi R, Wang Y, Nian M, Zhang J. Exposure to organophosphate esters and metabolic syndrome in adults. Environ Int. 2020;143:105941.
Bekele TG, Zhao H, Yang J, Chegen RG, Chen J, Mekonen S, et al. A review of environmental occurrence, analysis, bioaccumulation, and toxicity of organophosphate esters. Environ Sci Pollut Res Int. 2021;28:49507–28.
van der Veen I, de Boer J. Phosphorus flame retardants: properties, production, environmental occurrence, toxicity and analysis. Chemosphere. 2012;88:1119–53.
Meeker JD, Cooper EM, Stapleton HM, Hauser R. Urinary metabolites of organophosphate flame retardants: temporal variability and correlations with house dust concentrations. Environ health Perspect. 2013;121:580–5.
Sundkvist AM, Olofsson U, Haglund P. Organophosphorus flame retardants and plasticizers in marine and fresh water biota and in human milk. J Environ Monit. 2010;12:943–51.
Arvaniti OS, Kalantzi OI. Determinants of flame retardants in non-occupationally exposed individuals - a review. Chemosphere. 2021;263:127923.
Wei GL, Li DQ, Zhuo MN, Liao YS, Xie ZY, Guo TL, et al. Organophosphorus flame retardants and plasticizers: sources, occurrence, toxicity and human exposure. Environ Pollut. 2015;196:29–46.
Poma G, Sales C, Bruyland B, Christia C, Goscinny S, Van Loco J, et al. Occurrence of organophosphorus flame retardants and plasticizers (PFRs) in Belgian foodstuffs and estimation of the dietary exposure of the adult population. Environ Sci Technol. 2018;52:2331–8.
Ospina M, Jayatilaka NK, Wong LY, Restrepo P, Calafat AM. Exposure to organophosphate flame retardant chemicals in the U.S. general population: data from the 2013-2014 National Health and Nutrition Examination Survey. Environ Int. 2018;110:32–41.
Van den Eede N, Heffernan AL, Aylward LL, Hobson P, Neels H, Mueller JF, et al. Age as a determinant of phosphate flame retardant exposure of the Australian population and identification of novel urinary PFR metabolites. Environ Int. 2015;74:1–8.
Yanagisawa R, Koike E, Win-Shwe TT, Kawaguchi M, Takano H. The impact of oral exposure to low-dose tris(2-butoxyethyl) phosphate in allergic asthmatic mice. J Appl Toxicol. 2020;40:1498–510.
Yanagisawa R, Koike E, Win-Shwe TT, Kawaguchi M, Takano H. Impact of dietary exposure to low-dose tris(1,3-dichloro-2-propyl)phosphate in allergic asthmatic mice. Immunopharmacol Immunotoxicol. 2021;43:599–610.
Araki A, Bastiaensen M, Ait Bamai Y, Van den Eede N, Kawai T, Tsuboi T, et al. Associations between allergic symptoms and phosphate flame retardants in dust and their urinary metabolites among school children. Environ Int. 2018;119:438–46.
Araki A, Saito I, Kanazawa A, Morimoto K, Nakayama K, Shibata E, et al. Phosphorus flame retardants in indoor dust and their relation to asthma and allergies of inhabitants. Indoor Air. 2014;24:3–15.
Bi C, Maestre JP, Li H, Zhang G, Givehchi R, Mahdavi A, et al. Phthalates and organophosphates in settled dust and HVAC filter dust of U.S. low-income homes: association with season, building characteristics, and childhood asthma. Environ Int. 2018;121:916–30.
Canbaz D, van Velzen MJ, Hallner E, Zwinderman AH, Wickman M, Leonards PE, et al. Exposure to organophosphate and polybrominated diphenyl ether flame retardants via indoor dust and childhood asthma. Indoor Air. 2016;26:403–13.
Bulkhi AA, Shepard KV 2nd, Casale TB, Cardet JC. Elevated testosterone is associated with decreased likelihood of current asthma regardless of sex. J Allergy Clin Immunol Pract. 2020;8:3029.e4–35.e4.
Haggerty CL, Ness RB, Kelsey S, Waterer GW. The impact of estrogen and progesterone on asthma. Ann Allergy Asthma Immunol. 2003;90:284–91.
Han YY, Forno E, Celedón JC. Vitamin D insufficiency and asthma in a US nationwide study. J Allergy Clin Immunol Pract. 2017;5:790.e1–6.e1.
Chen TC, Parker JD, Clark J, Shin HC, Rammon JR, Burt VL. National Health and Nutrition Examination Survey: Estimation Procedures, 2011-2014. Vital Health Stat 2. 2018;1–26.
Jayatilaka NK, Restrepo P, Williams L, Ospina M, Valentin-Blasini L, Calafat AM. Quantification of three chlorinated dialkyl phosphates, diphenyl phosphate, 2,3,4,5-tetrabromobenzoic acid, and four other organophosphates in human urine by solid phase extraction-high performance liquid chromatography-tandem mass spectrometry. Anal Bioanal Chem. 2017;409:1323–32.
CDC. NHANES 2013-2014 questionnaire instruments: medical conditions. 2013. https://wwwn.cdc.gov/Nchs/Nhanes/Search/DataPage.aspx?Component=Questionnaire&CycleBeginYear=2013.
Lødrup Carlsen KC, Roll S, Carlsen KH, Mowinckel P, Wijga AH, Brunekreef B, et al. Does pet ownership in infancy lead to asthma or allergy at school age? Pooled analysis of individual participant data from 11 European birth cohorts. PLoS ONE. 2012;7:e43214.
Miller MR, Hankinson J, Brusasco V, Burgos F, Casaburi R, Coates A, et al. Standardisation of spirometry. Eur Respir J. 2005;26:319–38.
Quanjer PH, Stanojevic S, Cole TJ, Baur X, Hall GL, Culver BH, et al. Multi-ethnic reference values for spirometry for the 3-95-yr age range: the global lung function 2012 equations. Eur Respir J. 2012;40:1324–43.
American Thoracic Society, European Respiratory Society. ATS/ERS recommendations for standardized procedures for the online and offline measurement of exhaled lower respiratory nitric oxide and nasal nitric oxide, 2005. Am J Respir Crit Care Med. 2005;171:912–30.
Fuseini H, Newcomb DC. Mechanisms driving gender differences in asthma. Curr Allergy Asthma Rep. 2017;17:19.
Han YY, Forno E, Celedón JC. Sex steroid hormones and asthma in a nationwide study of U.S. adults. Am J Respir Crit Care Med. 2020;201:158–66.
Han YY, Forno E, Celedon JC. Adiposity, fractional exhaled nitric oxide, and asthma in U.S. children. Am J Respir Crit Care Med. 2014;190:32–9.
Institute of Medicine Committee to Review Dietary Reference Intakes for Vitamin D and Calcium. The National Academies Collection: reports funded by National Institutes of Health. In: Ross AC, Taylor CL, Yaktine AL, Del Valle HB, editors. Dietary reference intakes for calcium and vitamin D. Washington (DC): National Academy of Sciences; 2011.
Peters U, Dixon AE, Forno E. Obesity and asthma. J Allergy Clin Immunol. 2018;141:1169–79.
Kuczmarski RJ, Ogden CL, Guo SS, Grummer-Strawn LM, Flegal KM, Mei Z, et al. 2000 CDC growth charts for the United States: methods and development. Vital Health Stat 11. 2002:1–190.
Sun Y, Xia PF, Xie J, Mustieles V, Zhang Y, Wang YX, et al. Association of blood trihalomethane concentrations with asthma in U.S. adolescents: nationally representative cross-sectional study. Eur Respir J. 2021. https://doi.org/10.1183/13993003.01440-2021.
Madrigal JM, Persky V, Pappalardo A, Argos M. Association of heavy metals with measures of pulmonary function in children and youth: results from the National Health and Nutrition Examination Survey (NHANES). Environ Int. 2018;121:871–8.
Accordini S, Janson C, Svanes C, Jarvis D. The role of smoking in allergy and asthma: lessons from the ECRHS. Curr Allergy Asthma Rep. 2012;12:185–91.
Kit BK, Simon AE, Brody DJ, Akinbami LJ. US prevalence and trends in tobacco smoke exposure among children and adolescents with asthma. Pediatrics. 2013;131:407–14.
Sun Y, Gong X, Lin W, Liu Y, Wang Y, Wu M, et al. Metabolites of organophosphate ester flame retardants in urine from Shanghai, China. Environ Res. 2018;164:507–15.
Cequier E, Sakhi AK, Marcé RM, Becher G, Thomsen C. Human exposure pathways to organophosphate triesters - a biomonitoring study of mother-child pairs. Environ Int. 2015;75:159–65.
Zhao F, Kang Q, Zhang X, Liu J, Hu J. Urinary biomarkers for assessment of human exposure to monomeric aryl phosphate flame retardants. Environ Int. 2019;124:259–64.
Liu Y, Gong S, Ye L, Li J, Liu C, Chen D, et al. Organophosphate (OP) diesters and a review of sources, chemical properties, environmental occurrence, adverse effects, and future directions. Environ Int. 2021;155:106691.
Canbaz D, Logiantara A, van Ree R, van Rijt LS. Immunotoxicity of organophosphate flame retardants TPHP and TDCIPP on murine dendritic cells in vitro. Chemosphere. 2017;177:56–64.
Krivoshiev BV, Beemster GTS, Sprangers K, Blust R, Husson SJ. A toxicogenomics approach to screen chlorinated flame retardants tris(2-chloroethyl) phosphate and tris(2-chloroisopropyl) phosphate for potential health effects. J Appl Toxicol. 2018;38:459–70.
Hsiao HP, Lin MC, Wu CC, Wang CC, Wang TN. Sex-specific asthma phenotypes, inflammatory patterns, and asthma control in a cluster analysis. J Allergy Clin Immunol Pract. 2019;7:556.e5–67.e5.
Vink NM, Postma DS, Schouten JP, Rosmalen JG, Boezen HM. Gender differences in asthma development and remission during transition through puberty: the TRacking Adolescents’ Individual Lives Survey (TRAILS) study. J Allergy Clin Immunol. 2010;126:498.e1-6–504.e1-6.
Kynyk JA, Mastronarde JG, McCallister JW. Asthma, the sex difference. Curr Opin Pulm Med. 2011;17:6–11.
Sakai T, Furoku S, Nakamoto M, Shuto E, Hosaka T, Nishioka Y, et al. The soy isoflavone equol enhances antigen-specific IgE production in ovalbumin-immunized BALB/c mice. J Nutr Sci Vitaminol. 2010;56:72–6.
Straub RH. The complex role of estrogens in inflammation. Endocr Rev. 2007;28:521–74.
Girón-González JA, Moral FJ, Elvira J, García-Gil D, Guerrero F, Gavilán I, et al. Consistent production of a higher TH1:TH2 cytokine ratio by stimulated T cells in men compared with women. Eur J Endocrinol. 2000;143:31–6.
Riffo-Vasquez Y, Ligeiro de Oliveira AP, Page CP, Spina D, Tavares-de-Lima W. Role of sex hormones in allergic inflammation in mice. Clin Exp Allergy. 2007;37:459–70.
de Oliveira AP, Peron JP, Damazo AS, Franco AL, Domingos HV, Oliani SM, et al. Female sex hormones mediate the allergic lung reaction by regulating the release of inflammatory mediators and the expression of lung E-selectin in rats. Respir Res. 2010;11:115.
Pfeffer PE, Hawrylowicz CM. Vitamin D in asthma: mechanisms of action and considerations for clinical trials. Chest. 2018;153:1229–39.
Agrawal T, Gupta GK, Agrawal DK. Vitamin D supplementation reduces airway hyperresponsiveness and allergic airway inflammation in a murine model. Clin Exp Allergy. 2013;43:672–83.
Fischer KD, Hall SC, Agrawal DK. Vitamin D supplementation reduces induction of epithelial-mesenchymal transition in allergen sensitized and challenged mice. PLoS ONE. 2016;11:e0149180.
Meng Y, Xu X, Xie G, Zhang Y, Chen S, Qiu Y, et al. Alkyl organophosphate flame retardants (OPFRs) induce lung inflammation and aggravate OVA-simulated asthmatic response via the NF-кB signaling pathway. Environ Int. 2022;163:107209.
Dixon AE, Holguin F, Sood A, Salome CM, Pratley RE, Beuther DA, et al. An official American Thoracic Society Workshop report: obesity and asthma. Proc Am Thorac Soc. 2010;7:325–35.
Forno E, Acosta-Pérez E, Brehm JM, Han YY, Alvarez M, Colón-Semidey A, et al. Obesity and adiposity indicators, asthma, and atopy in Puerto Rican children. J Allergy Clin Immunol. 2014;133:1308–14.
Boyle M, Buckley JP, Quirós-Alcalá L. Associations between urinary organophosphate ester metabolites and measures of adiposity among U.S. children and adults: NHANES 2013-2014. Environ Int. 2019;127:754–63.
Forno E. Childhood obesity and asthma: to BMI or not to BMI? J Allergy Clin Immunol. 2017;139:767–8.
Xu F, Eulaers I, Alves A, Papadopoulou E, Padilla-Sanchez JA, Lai FY, et al. Human exposure pathways to organophosphate flame retardants: associations between human biomonitoring and external exposure. Environ Int. 2019;127:462–72.
Acknowledgements
The authors thank all participants in the NHANES for making this study possible.
Funding
This study was funded by the National Key R&D Program of China (2022YFC3902100) and the Discipline Construction Project of the School of Population Medicine and Public Health, Chinese Academy of Medical Sciences and Peking Union Medical College (2021-EFT-Z-016).
Author information
Authors and Affiliations
Contributions
RA and CD completed data analyses and contributed to the writing of the manuscript. ZX prepared the NHANES data for analysis. YQ reviewed manuscript and provided critical comments. YJ and KL critically revised and edited the manuscripts. All authors approved the final version of the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval and consent to participate
The study protocols were approved by the institutional review board of the National Center for Health Statistics and written informed consents were obtained from all participants.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Aimuzi, R., Dong, C., Xie, Z. et al. Associations of urinary organophosphate esters metabolites with asthma and lung function in adolescents. J Expo Sci Environ Epidemiol (2023). https://doi.org/10.1038/s41370-023-00540-2
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41370-023-00540-2