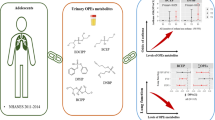
Abstract
Background
Environmental exposures such as perfluoroalkyl substances (PFASs) were considered potential risks for bone mineral density (BMD).
Objective
To examine the associations between PFASs and BMD among the U.S. population.
Methods
This study included a total of 6416 participants from the National Health and Nutrition Examination Survey (NHANES 2005–2014). Multiple linear regression models were used to analyze the associations between serum PFASs and BMD and the coefficient β with 95% confidence intervals (95% CI) was calculated as the effect estimate. Covariates such as age, race, BMI, smoking, alcohol intake, milk intake, and physical activity were adjusted in these models. Additionally, gender and menopausal period were considered in further subgroup analyses.
Results
Based on the combined data of NHANES 2005–2014, the effects from exposure to PFASs on BMD were found with gender and menopausal status differences. Positive associations were found in PFOA (β = 0.010; 95% CI: 0.003, 0.016), PFHxS (β = 0.007; 95% CI: 0.003, 0.012), and PFNA (β = 0.001; 95% CI: 0.001, 0.017) in total population. Negative associations for PFOA (β = −0.020; 95% CI: −0.029, −0.012), PFOS (β = −0.011; 95% CI: −0.028, −0.011), PFHxS (β = −0.019; 95% CI: −0.025, −0.013), PFDE (β = −0.010; 95% CI: −0.016, −0.005), and PFNA (β = −0.011; 95% CI: −0.021, −0.002) were found in women, while no significant association was found in men. In further subgroup analyses, women in pre-menopause status showed consistent negative associations.
Significance
PFASs exposure may be associated with BMD and gender and menopausal status confound the associations.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 6 print issues and online access
$259.00 per year
only $43.17 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
French ZP, Caird MS, Whitney DG. Osteoporosis epidemiology among adults with cerebral palsy: findings from private and public administrative claims data. JBMR. 2019;11:e10231.
Guo J, Huang Y, Bian S, Zhao C, Jin Y, Yu D, et al. Associations of urinary polycyclic aromatic hydrocarbons with bone mass density and osteoporosis in U.S. adults, NHANES 2005-2010. Environ Pollut. 2018;240:209–18.
Cai S, Zhu J, Sun L, Fan C, Zhong Y, Shen Q, et al. Association between urinary triclosan with bone mass density and osteoporosis in US adult women, 2005-2010. J Clin Endocrinol Metab. 2019;10:4531–8.
O’Carroll DM, Jeffries TC, Lee MJ, Le ST, Yeung A, Wallace S, et al. Developing a roadmap to determine per- and polyfluoroalkyl substances-microbial population interactions. Sci Total Environ. 2020;712:135994.
Suominen K, Hallikainen A, Ruokojarvi P, Airaksinen R, Koponen J, Rannikko R, et al. Occurrence of PCDD/F, PCB, PBDE, PFAS, and organotin compounds in fish meal, fish oil and fish feed. Chemosphere. 2011;3:300–6.
Flynn RW, Chislock MF, Gannon ME, Bauer SJ, Tornabene BJ, Hoverman JT, et al. Acute and chronic effects of perfluoroalkyl substance mixtures on larval American bullfrogs (Rana catesbeiana). Chemosphere. 2019;236:124350.
Dennis NM, Karnjanapiboonwong A, Subbiah S, Rewerts JN, Field JA, McCarthy C, et al. Chronic reproductive toxicity of perfluorooctane sulfonic acid and a simple mixture of perfluorooctane sulfonic acid and perfluorohexane sulfonic acid to Northern Bobwhite Quail (Colinus virginianus). Environ Toxicol Chem. 2020;39:1101–11.
Fernandez-Sanjuan M, Faria M, Lacorte S, Barata C. Bioaccumulation and effects of perfluorinated compounds (PFCs) in zebra mussels (Dreissena polymorpha). Environ Sci Pollut Res Int. 2013;4:2661–9.
Caron-Beaudoin E, Ayotte P, Laouan SE, Gros-Louis MN, Lemire M. Exposure to perfluoroalkyl substances (PFAS) and associations with thyroid parameters in First Nation children and youth from Quebec. Environ Int. 2019;128:13–23.
Worley RR, Moore SM, Tierney BC, Ye X, Calafat AM, Campbell S, et al. Per- and polyfluoroalkyl substances in human serum and urine samples from a residentially exposed community. Environ Int. 2017;106:135–43.
Francisca P, Marti N, Alicia NO, Francesc F, Jose LD, Damia B, et al. Accumulation of perfluoroalkyl substances in human tissues. Environ Int. 2013;59:354–62.
Bassler J, Ducatman A, Elliott M, Wen S, Wahlang B, Barnett J, et al. Environmental perfluoroalkyl acid exposures are associated with liver disease characterized by apoptosis and altered serum adipocytokines. Environ Pollut. 2019;247:1055–63.
Jacquet N, Maire MA, Rast C, Bonnard M, Vasseur P. Perfluorooctanoic acid (PFOA) acts as a tumor promoter on Syrian hamster embryo (SHE) cells. Environ Sci Pollut Res Int. 2011;7:2537–49.
Sammi SR, Foguth RM, Nieves CS, De Perre C, Wipf P, McMurray CT, et al. Perfluorooctane sulfonate (PFOS) produces dopaminergic neuropathology in Caenorhabditis elegans. Toxicol Sci. 2019;2:417–34.
Zarei MH, Hosseini SS, Aghvami M, Pourahmad J. Perfluorooctanesulfonate (PFOS) induces apoptosis signaling and proteolysis in human lymphocytes through ROS mediated mitochondrial dysfunction and lysosomal membrane labialization. Iran J Pharm Res. 2018;3:995–1007.
Fenton SE, Ducatman A, Boobis A, DeWitt JC, Lau C, Ng C, et al. Per- and polyfluoroalkyl substance toxicity and human health review: current state of knowledge and strategies for informing future research. Environ Toxicol Chem. 2020;40:606–30.
Hu Y, Liu G, Rood J, Liang L, Bray GA, de Jonge L, et al. Perfluoroalkyl substances and changes in bone mineral density: a prospective analysis in the POUNDS-LOST study. Environ Res. 2019;179:108775.
Di Nisio A, De Rocco PM, Giadone A, Rocca MS, Guidolin D, Foresta C. Perfluoroalkyl substances and bone health in young men: a pilot study. Endocrine. 2020;3:678–84.
Lind PM, Lind L, Salihovic S, Ahlstrom H, Michaelsson K, Kullberg J. et al. Serum levels of perfluoroalkyl substances (PFAS) and body composition—a cross-sectional study in a middle-aged population. Environ Res. 2022;209:112677.
Calafat AM, Kuklenyik Z, Reidy JA, Caudill SP, Tully JS, Needham LL. Serum concentrations of 11 polyfluoroalkyl compounds in the u.s. population: data from the national health and nutrition examination survey (NHANES). Environ Sci Technol. 2007;7:2237–42.
Holzer J, Midasch O, Rauchfuss K, Kraft M, Reupert R, Angerer J, et al. Biomonitoring of perfluorinated compounds in children and adults exposed to perfluorooctanoate-contaminated drinking water. Environ Health Perspect. 2008;5:651–7.
Kudo N, Kawashima Y. Toxicity and toxicokinetics of perfluorooctanoic acid in humans and animals. J Toxicol Sci. 2003;2:49–57.
Fromme H, Tittlemier SA, Volkel W, Wilhelm M, Twardella D. Perfluorinated compounds-exposure assessment for the general population in Western countries. Int J Hyg Environ Health. 2009;3:239–70.
Lin LY, Wen LL, Su TC, Chen PC, Lin CY. Negative association between serum perfluorooctane sulfate concentration and bone mineral density in US premenopausal women: NHANES, 2005-2008. J Clin Endocrinol Metab. 2014;6:2173–80.
Khalil N, Chen A, Lee M, Czerwinski SA, Ebert JR, DeWitt JC, et al. Association of perfluoroalkyl substances, bone mineral density, and osteoporosis in the U.S. population in NHANES 2009-2010. Environ Health Perspect. 2016;1:81–7.
Cluett R, Seshasayee SM, Rokoff LB, Rifas-Shiman SL, Ye X, Calafat AM, et al. Per- and polyfluoroalkyl substance plasma concentrations and bone mineral density in midchildhood: a cross-sectional study (Project Viva, United States). Environ Health Perspect. 2019;8:87006.
Kang JS, Ahn TG, Park JW. Perfluorooctanoic acid (PFOA) and perfluooctane sulfonate (PFOS) induce different modes of action in reproduction to Japanese medaka (Oryzias latipes). J Hazard Mater. 2019;368:97–103.
Behr AC, Lichtenstein D, Braeuning A, Lampen A, Buhrke T. Perfluoroalkylated substances (PFAS) affect neither estrogen and androgen receptor activity nor steroidogenesis in human cells in vitro. Toxicol Lett. 2018;291:51–60.
Pierozan P, Cattani D, Karlsson O. Perfluorooctane sulfonate (PFOS) and perfluorooctanoic acid (PFOA) induce epigenetic alterations and promote human breast cell carcinogenesis in vitro. Arch Toxicol. 2020;11:3893–906.
Zhang L, Meng Z, Chen L, Zhang G, Zhang W, Tian Z, et al. Perfluorooctanoic acid exposure impact a trade-off between self-maintenance and reproduction in lizards (Eremias argus) in a gender-dependent manner. Environ Pollut. 2020;262:114341.
Kim SJ, Heo SH, Lee DS, Hwang IG, Lee YB, Cho HY. Gender differences in pharmacokinetics and tissue distribution of 3 perfluoroalkyl and polyfluoroalkyl substances in rats. Food Chem Toxicol. 2016;97:243–55.
Zhu JH, Liao YP, Li FS, Hu Y, Li Q, Ma Y, et al. Wnt11 promotes BMP9-induced osteogenic differentiation through BMPs/Smads and p38 MAPK in mesenchymal stem cells. J Cell Biochem. 2018;11:9462–73.
Jain RB. Association between thyroid profile and perfluoroalkyl acids: data from NHNAES 2007-2008. Environ Res. 2013;126:51–9.
Black DM, Rosen CJ. Clinical practice. Postmenopausal osteoporosis. N Engl J Med. 2016;3:254–62.
Jesserer H. [Fluorine therapy of osteoporosis]. Hippokrates. 1974;3:354–65.
Liu S, Zhou H, Liu H, Ji H, Fei W, Luo E. Fluorine-contained hydroxyapatite suppresses bone resorption through inhibiting osteoclasts differentiation and function in vitro and in vivo. Cell Prolif. 2019;3:e12613.
Koskela A, Koponen J, Lehenkari P, Viluksela M, Korkalainen M, Tuukkanen J. Perfluoroalkyl substances in human bone: concentrations in bones and effects on bone cell differentiation. Sci Rep. 2017;1:6841.
Yamamoto J, Yamane T, Oishi Y, Kobayashi-Hattori K. Perfluorooctanoic acid binds to peroxisome proliferator-activated receptor gamma and promotes adipocyte differentiation in 3T3-L1 adipocytes. Biosci Biotechnol Biochem. 2015;4:636–9.
Taniguchi IE, Chang KH, Nayak R, Olsson HA, Ficker AM, Dunn SK, et al. Klf5 controls bone marrow homing of stem cells and progenitors through Rab5-mediated beta1/beta2-integrin trafficking. Nat Commun. 2013;4:1660.
Chen Z, Zhang Q, Wang H, Li W, Wang F, Wan C, et al. Klf5 mediates odontoblastic differentiation through regulating dentin-specific extracellular matrix gene expression during mouse tooth development. Sci Rep. 2017;7:46746.
Lee SA, Choi JY, Shin CS, Hong YC, Chung H, Kang D. SULT1E1 genetic polymorphisms modified the association between phytoestrogen consumption and bone mineral density in healthy Korean women. Calcif Tissue Int. 2006;3:152–9.
Acknowledgements
Special appreciation should be given to the NHANES team and the participants in it.
Funding
This study was supported by Natural Science Foundation of Zhejiang Province (LY20H170002).
Author information
Authors and Affiliations
Contributions
XZ: conceptualization, statistical analysis, methodology, manuscript development, funding acquisition; JYL: data analysis, manuscript review; WWD: data analysis, manuscript review; MLT: statistical analysis, methodology, manuscript revision; SGY: conceptualization, supervision, manuscript review.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethical approval
The data in this article come from NHANES. No Ethics approval and consent to participate is needed.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Zhao, X., Lin, JY., Dong, WW. et al. Per- and polyfluoroalkyl substances exposure and bone mineral density in the U.S. population from NHANES 2005–2014. J Expo Sci Environ Epidemiol 33, 69–75 (2023). https://doi.org/10.1038/s41370-022-00452-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41370-022-00452-7