Abstract
The crosstalk between the nerve and stomatognathic systems plays a more important role in organismal health than previously appreciated with the presence of emerging concept of the “brain-oral axis”. A deeper understanding of the intricate interaction between the nervous system and the stomatognathic system is warranted, considering their significant developmental homology and anatomical proximity, and the more complex innervation of the jawbone compared to other skeletons. In this review, we provide an in-depth look at studies concerning neurodevelopment, craniofacial development, and congenital anomalies that occur when the two systems develop abnormally. It summarizes the cross-regulation between nerves and jawbones and the effects of various states of the jawbone on intrabony nerve distribution. Diseases closely related to both the nervous system and the stomatognathic system are divided into craniofacial diseases caused by neurological illnesses, and neurological diseases caused by an aberrant stomatognathic system. The two-way relationships between common diseases, such as periodontitis and neurodegenerative disorders, and depression and oral diseases were also discussed. This review provides valuable insights into novel strategies for neuro-skeletal tissue engineering and early prevention and treatment of orofacial and neurological diseases.
Similar content being viewed by others
Introduction
With the advancement of brain science in recent years, the association between the nervous system and the stomatognathic system has become increasingly evident. To this effect, new concepts, such as neuromuscular dentistry1,2 and stomatopsychology3 have been proposed to explain the interaction between the two systems. Additionally, research has highlighted the importance of nerves in craniomaxillofacial development,4 as well as the crosstalk between nerves and jawbone,5 and the diseases that can arise from them.
Anatomically, the nervous and stomatognathic systems are evidently close in proximity. The nervous system consists of the central nervous system (CNS) and the peripheral nervous system (PNS). The former includes the brain and spinal cord, and the latter comprises cranial nerves (linking with the brain) and spinal nerves (linking with the spinal cord).6 The peripheral nerves associated with the oral and maxillofacial development region include the trigeminal nerve, facial nerve, glossopharyngeal nerve, vagus nerve, accessory nerve, hypoglossal nerve and even cervical spinal nerves.7 The nervous system regulates the stomatognathic system in a variety of ways, from maxillofacial bones to dental pulp, periodontal ligament (PDL), muscles, glands, oral mucosa, the tongue, the temporomandibular joint (TMJ), mouth, skin, and other structures.8 This intricate regulation of the nervous system is vital for the proper development and functioning of the maxillofacial system. Maxillofacial deformity and skeletal dysplasia are common comorbidities in neurodevelopmental deficit patients, such as trisomy 21 (ref. 9), neurofibromatosis,10 and achondroplasia.11
The regulation between nerves and bones has been widely studied,12 with intrabony nerves being found in cortical bone,8 trabecular bone, periosteum, and bone marrow.13,14 The CNS regulates bone metabolism through the peripheral autonomic nervous system (ANS) and sensory nerves. The ANS comprises the sympathetic nervous system (SNS) and the parasympathetic nervous system (PSNS).15,16 All peripheral nerves regulate bone development and recover via neurotransmitters, neuropeptides, neurotrophins, and others.17 In the case of the jawbone, nerves not only distribute in the same parts as other bones, but also in special parts, such as the subchondral condyle, PDL, and dental pulp.18 In addition to classic targets, such as osteoclasts and osteoblasts, these parts are also targets of the nervous system that mediates jawbone remodeling. The regulation of nerves on the oral and maxillofacial systems is unique and significant due to the presence of more targets. Furthermore, because of the special anatomy of the jawbone—branches of the trigeminal nerve travel in the intraosseous canals and innervate peripheral tissues,19 concomitant peripheral nerve injury can be caused by jawbone defects, and bone repair is accompanied by nerve repair.20
The proximity of anatomical structures, and the rich circulatory system of the brain and maxillofacial region, enable the nervous system and the stomatognathic system to interact with each other. The decline or loss of neurological function can result in some oral symptoms, such as facial paralysis21 and salivation.22 Conversely, oral diseases can influence the nervous system. If oral bacteria intrude into the brain via hematogenous spread, caries, periodontitis, and other oral infections may lead to intracranial infection and even neurodegenerative and neuropsychological diseases.23 Oral squamous cell carcinoma (OSCC) and adenoid cystic carcinoma (ACC) can lead to perineural invasion (PNI) of the head and neck as well, resulting in numbness, pain, or dysfunction.24 More importantly, the mechanism of some systemic diseases, such as Alzheimer’s disease (AD) and Parkinson’s disease (PD), are too complex to recognize their initiating lesions. Some nervous system diseases and stomatognathic diseases can promote each other, such as depression and periodontal disease,25 and pain caused by neuropathy and stomatognathic lesions.26 Although abnormalities in the stomatognathic system are not the major cause of neurological diseases, it is important to note that the abnormalities can contribute to their progression. Therefore, understanding the potential links between these two systems is essential for early diagnosis and improved prognosis.
This review provides a comprehensive analysis of the cellular and molecular regulatory mechanisms between nerves and maxillofacial cells during growth and in both physiological and abnormal environments. It further examines the development of the oral and maxillofacial systems, wound healing, and other visible changes from a macro perspective. Additionally, it summarizes the nervous system diseases and disorders caused by the oral and maxillofacial systems, as well as the complex diseases that are strongly linked to the interaction between the nervous and stomatognathic systems. By gaining a better understanding of these complex scenarios, we can further investigate the underlying mechanisms and apply them to clinical settings for the early prevention and treatment of diseases in the future.
The physiological growth and developmental anomalied of nervous and craniomaxillofacial systems
Physiological growth of nervous and craniomaxillofacial systems
It has been reported that cranial and maxillofacial development in vertebrates is closely related to neural growth.4 During this process, neural crest (NC) cells play a pivotal role, which are characterized by their multi-potential, migration, and differentiation abilities. In early embryonic development, NC cells first appear on the dorsal side of the neural tube and initiate the expression of NC signature genes (FoxD3, Sox10, etc.), signifying the formation of true NC cells.27,28 Subsequently, NC cells undergo an epithelial-to-mesenchymal transition to migrate extensively during the entire embryonic development. NC cells can be divided into four main groups along the cephalic and caudal axis: cranial, vagal, trunk, and sacral ganglion subgroups.29 Among them, cranial neural crest (CNC) cells, derived from labeling NC cells with Wnt1, are the most significant group involved in craniofacial development, and the only group related to cranial bone formation.30 The migration of CNC cells is highly regulated and occurs along well-defined pathways, terminating in the ventral part of the brain and the branchial arch. CNC cells first migrate as continuous waves and rapidly split into three discrete streams to fill the first, second and third branchial arches. Subsequently, CNC cells contribute to various structures, including the skeletal system (cartilage and jawbone), cranial nerves and ganglia, as well as smooth muscle, vascular connective tissue, and the dermis of the head.31 Moreover, CNC cells form multiple components of the tooth through sequential and induced epithelial-mesenchymal interactions between odontogenic mesenchymal cells derived from CNC and the covering ectoderm.32 Consequently, nerves play a crucial role in cranial and maxillofacial development.
Developmental anomalies of the nervous and craniomaxillofacial systems
There are many congenital or genetic diseases that have multiple concurrent developmental alterations affecting the nervous system and stomatognathic system, some definitely serious for survival and others with less dramatic prognoses for life. Here are three of the typical diseases, and Table 1 lists additional ones.
Trisomy 21
Trisomy 21 (Down syndrome) is a genetic disorder resulting from an extra copy of human chromosome 21, occurring at a frequency of 1:600 to 1:2 000 (ref. 33). In fact, abnormal expression of non‑HSA21 genes and deregulated non-coding genetic elements also influences brain and cognitive development in Trisomy 21. Patients with Trisomy 21 often suffer from mental retardation, neurodevelopmental disorders, and even AD with age.9 They typically exhibit deficits in short-term memory and language abilities, as well as a variety of oral symptoms such as periodontitis, angular lip cheilitis,34 missing teeth, malformed teeth, delayed tooth eruption, malocclusion, fissured lips and tongue, macroglossia, mouth breathing, and bruxism.35 The etiology of hypodontia abnormal development of the teeth may refer to alterations in the PNS36 or the abnormalities in tooth germs.37 Inflammation, on the other hand, can be linked to alterations in patients’ immune response38 or various systemic or infectious diseases.39 Although novel treatments are being investigated, treatment of Trisomy 21 is largely based on approaches used for other diseases, such as AD.40 And craniofacial or dentoalveolar aesthetics of patients with Trisomy 21 can be improved with surgical procedures and orthodontic treatments.35,41
Neurofibromatosis type 1
Many reports have demonstrated concomitant morpho-functional alteration in the stomatognathic system in individuals with neurofibromatosis. Neurofibromatosis is divided into two types: type 1 and type 2, the more common being Neurofibromatosis type 1 (NF1), which occurs at a frequency of 1 in 1000. NF1 is an autosomal dominant inherited disorder, and its pathogenesis is associated with mutations of the NF1 gene, which encodes the tumor suppressor neurofibromin.42,43 These mutations lead to the hyperactivation of the rat sarcoma mitogen-activated protein kinase (RAS-MAPK) pathway, which provokes cell hyperproliferation or tumorigenesis, like neurofibromas, optic pathway gliomas, astrocytomas, and malignant peripheral nerve sheath tumors.10 Because NF1 affects the underlying facial skeleton and can even directly infiltrate or pull down surrounding tissues, midface deformity is common in NF1 patients.44 Oral manifestations can be found in approximately 72% of NF1 patients,45 with hard tissue (jawbone and teeth) malformations like intrabony cystic lesions, enlarged or branched mandibular canals46 and malocclusion remaining prominent across the board.47 In addition, soft tissue deformities are frequently seen due to the morphological variations in particular sites. Examples of such deformities include malformed nose and upper lip areas, gingival enlargement,48 gingival neurofibroma,45 nodular lesions on the tongue,49 and perineural fibrous thickening within the dental pulp.50 Due to a broad spectrum of lesions associated with NF1, surgical resection is usually used for therapy44
Achondroplasia
The formation of mammalian skeletons occurs via intramembranous or endochondral ossification. The former occurs in the midface and the latter occurs in the skull base and nasal septum.51 Achondroplasia is the most prevalent genetic disorder of dwarfism, occurring at a frequency of 1 in 26,000 (ref. 52). Its pathogenesis is linked to activating mutations in the gene encoding fibroblast growth factor receptor 3 (FGFR3),53 which is a pivotal regulator of endochondral bone growth. Activated FGFR3 signaling in chondrocytes increases the expression of Bmp ligand mRNA, which promotes osteoblast differentiation and accelerates bone formation and synchondrosis closure. Furthermore, the early closure of synchondroses may lead to the narrowing of the foramen magnum and spinal canals,54 resulting in severe neurological complications, including radiculopathy, myelopathy, and neurogenic claudication. In terms of maxillofacial symptoms, achondroplasia patients may have a prominent forehead, midface hypoplasia, occlusal abnormality, low nose bridge, narrow nasal passages, all of which are caused by defective endochondral ossification in craniofacial cartilage and premature closure of the growth center in craniomaxillofacial skeletogenesis.11 Due to critical illness in the nervous and orofacial system, any intervention ought to be implemented before the synchondrosis closure.
Homestasis and regulation between the nervous system and jawbones
Effect of nerves on jawbones
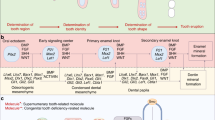
The anatomical structure of the jawbone is unique: the nerves travel in the bony ducts and send branches directly to surrounding tissues. The trigeminal nerve, the largest cranial nerve, comprises the ophthalmic, maxillary, and mandibular branches.55 The maxillary nerve innervates the maxilla, and the inferior alveolar nerve (IAN), which is the largest branch of the mandibular nerve, innervates the mandible.56 In addition to branches of the trigeminal nerve, ANS also plays a significant role in the physiology and pathology of the jawbone.57 Experimental animal studies have shown that the complex and intricate mechanism involves various nerves and bioactive factors secreted within the microenvironment.57,58 In particular, intrabony nerves regulate jawbone metabolism through neurotransmitters, neuropeptides, neurotrophins, and other signaling molecules.59,60 The tyrosine-hydroxylase-immunoreactive (TH-IR) fibers and vasoactive intestinal polypeptide (VIP)-IR fibers are sympathetic fibers. The TH-IR and VIP-IR fibers secrete norepinephrine (NE) and VIP respectively. Sensory neurons secrete calcitonin gene-related peptide (CGRP) and substance P (SP), so CGRP-IR fibers and SP-IR fibers are sensory fibers.61 The accumulation of various biological factors within the microenvironment of jawbones, along with the presence of their receptors in osteogenic and osteoblast lineage cells,62,63,64 provides compelling evidence of bilateral homeostasis between nerves and the jawbone (Table 2 and Fig. 1).
Effect of nerves on jawbones. a Effect of ANS and sensory nerves on jawbones. VIP and NE released from sympathetic nerves can activate corresponding receptors and upregulate RANKL in OBs, and RANKL contributes to OC maturation. All of them lead to bone resorption. Ach released from parasympathetic nerves may contribute to anti-inflammatory activity and osteoclast apoptosis. CGRP and SP released from sensory nerves downregulate RANKL and upregulate OPG in OBs, thereby hastening bone formation. b The neurofeedback in the PDL under the induction of orthodontic force. Orthodontic force triggers nociceptors in sensory fibers, leading to inflammatory cascade mediated by CGRP and SP, as well as the activated neural loop of the sensory-central-SNS. Orthodontic force also activates sympathetic nerves and promotes osteoclast activity. The neurofeedback influences alveolar bone remodeling and tooth movement. PGE 2 prostaglandin E2, IL-6 interleukin-6, Ach acetylcholine, NK1R neurokinin 1 receptor, CRL+RAMP1 calcitonin receptor-like receptor+receptor activity-modifying protein 1, V trigeminal nerve
Autonomic nervous system
Animal experiments show that SNS negatively affects bone mass,65 whereas PSNS does the opposite.66 Previous research indicates that heightened SNS activity causes bone loss.67 SNS promotes bone resorption through the released NE and active β2-adrenergic receptors (β2-ARs),62 as well as the receptor activator of nuclear factor kappa B ligand (RANKL)—osteoprotegerin (OPG) system.68 The impact of SNS on the jawbone is more complicated than previously reported. Both TH-IR fibers and VIP-IR fibers distribute within the mandible periosteum and alveolar wall, but the distribution of TH-IR fibers is wider, and includes the mandibular endosteal retromolar zone. NE and VIP are two bioactive factors that contribute to osteoclast differentiation and bone resorption. Following sympathectomy, the number of TH-IR fibers and VIP-IR fibers declines, while the number of CGRP-IR fibers increases,61 which is associated with sensory-sympathetic interactions mediated by neurotrophic factors.69 Sympathectomy changes the expression of NGF and semaphorin 3A (sema3a), leading to the increase of CGRP-IR fibers.70 Following a superior cervical ganglionectomy in female rats, bone mineral density increased significantly.57 This can be attributed to the inhibition of the SNS, which decreases the number of RANKL-expressing osteoblasts and preosteoclasts in the mandibular periosteum, thereby facilitating osteogenesis.5 Nerve fibers also innervate the TMJ, and active sympathetic signaling has been found to be related to bone loss during osteoarthritis of the TMJ, whereas the use of β2-ARs antagonists can suppress subchondral bone resorption and osteoclast function.71 Therefore, the metabolism of different regions of the jawbone is modulated by the sympathetic pathways.
In addition, the relationship between ANS and immune response has been investigated in the alveolar bone.72 Acetylcholine (a neurotransmitter secreted by PSNS) and its receptors have been found to be expressed in various non-neuronal cells including human keratinocytes,73 fibroblasts, T cells, B cells and macrophages.74,75 Clinical data and animal experiments reveals that acetylcholine can regulate inflammation-related cells by activating the α7 nicotinic receptor, which promotes anti-inflammatory activity75 and reduces the release of inflammatory factors.76,77,78 In fact, PSNS activation can promote osteoclast apoptosis to favor bone mass accrual.66 It has been found that electrical activation of the carotid sinus nerve can alleviate alveolar bone loss and periodontal disease in rats. This effect may be attributed to activation of PSNS and its anti-inflammatory response by provoking baroreflex and chemoreflex.72 However, comprehensive and thorough research investigating the regulation of ANS on the jawbone is relatively scarce. Therefore, further exploration is needed to understand the effect of ANS on the jawbone and its underlying mechanism.
Sensory nerves
The role of sensory nerves should not be ignored in bone regeneration. At the micro‐level, these nerves promote bone recovery through the release of neuropeptides, such as CGRP and SP. Their receptors are expressed on bone cells,5,63,64 indicating a strong association between the nervous system and bone metabolism in animal models. CGRP is a positive mediator for bone modeling, as it suppresses the number of osteoclasts by regulating the OPG/RANKL ratio. CGRP also promotes the osteogenic differentiation of human PDL stem cells to repair rat alveolar bone defects.79 However, the effect of SP appears to be contradictory. In vitro, studies indicate that SP can stimulate osteoblast and osteoclast differentiation and function.80 In vivo, studies show that a combination of SP and calcium phosphate cement can contribute to alveolar bone defect restoration.81 Additionally, SP has been found to hasten bone formation during mandibular distraction osteogenesis.82 Nonetheless, SP can inhibit osteogenesis induced by lipopolysaccharide from Porphyromonas gingivalis.64 Generally, CGRP and SP act synergistically since they are frequently co-localized in the same fibers and bone defect sites and released synergistically. After transection of the IAN, the secretion of CGRP and SP decreases,58,59 which reduces the OPG/RANKL ratio and promotes osteoclastogenesis. Thus, injured or transected IAN result in sensory nerve degradation and mandibular bone destruction. Nerve growth factor (NGF), a key neurotrophin released by sympathetic and sensory nerves,83,84 has been found to stimulate bone formation by inducing regenerating axons,85 and consequently, improving the density and quality of new bone in a rabbit model of mandibular distraction osteogenesis.86 Altogether, these findings indicate that sensory nerves play a significant role in bone formation and regeneration (Fig. 1a).
In addition to the classical pathways of neural regulation, such as those of limb bones, jawbone remodeling is also regulated by neural signals within the PDL.87 The PDL is the soft tissue between the teeth and alveolar bone, and it serves as a critical anatomical structure in orthodontic treatment. It has been reported that fibroblasts and osteoblasts in the PDL may respond directly to mechanical forces and initiate the remodeling of alveolar bone88,89 through mechanotransduction90,91 and intracellular signaling cascades.92,93 Additionally, the PDL is abundantly supplied with sympathetic, parasympathetic and sensory fibers,94,95 which contribute to alveolar bone remodeling and tooth movement. As mentioned before, sympathetic fibers release NE and VIP to promote bone resorption, while parasympathetic fibers secret acetylcholine to inhibit bone resorption.66 Thinly myelinated and unmyelinated sensory fibers express CGRP and SP to facilitate osteogenesis.87 Sensory fibers in the PDL contain nociceptors,96 which are triggered by orthodontic force, resulting in transmission of painful signals to the brain.97,98,99 This process activates an inflammatory cascade in the trigeminal spinal nucleus.87 It is mediated by the activation of neurons and inflammatory cells,100,101 leading to an increase in the secretion of NGF,102 CGRP,103 SP104 and various inflammatory molecules.87 In addition, the activated neural loop of the sensory-central-SNS influences orthodontic tooth movement.105 In summary, the PDL is a complex system, and nerves within it play a critical role in tooth movement and alveolar bone remodeling (Fig. 1b).
Regulation of jawbones to nerves
The condition of the jawbone can also affect the distribution of nerves.
Anatomical factors
The presence of teeth and the intraosseous canal makes the jawbone unique compared to other bones, and also affects nerve distribution. The mandibular canal is a compact bone canal in the cancellous bone of the mandible. The IAN runs through the mandibular canal and sends branches to control the teeth in what are known as mandibular canal branches. The number of these mandibular canal branches is largely determined by the number of teeth and occlusion elements in the human mandible.106 Since the presence of teeth helps to maintain the alveolar bone matrix,107 when teeth are lost, nerve branches may disappear due to the absorption of alveolar bone.106,108
Mechanical factors
Actually, nerves can sense and respond to mechanical signals, which include the rigidity of the environment and press/traction exerted on the neurons by neighboring cells.109 The latter signal includes the tension of the jawbone and the orthodontic force of the teeth. After mandibular distraction osteogenesis, the elongation of the IAN occurs along with mandible regeneration in dogs.110 Aside from traction on the mandible, the orthodontic force on the teeth can also affect the distribution of nerves in the PDL, which is a specialized fibrous connective tissue, and dental pulp, which is connected to the PDL through the dentinal tubules and apical foramen. Dental pulp and PDL are richly supplied with sensory and sympathetic nerve fibers. They also feature immunoreactivity to protein gene product 9.5 and CGRP.95,111 It has been demonstrated that the reaction of the PDL is directly related to the duration, type, direction, and magnitude of the force on the teeth.112,113 Appropriate and intermittent orthodontic force will not cause permanent damage for the PDL and pulp.114 The density of nerve fibers in the pulp and PDL increases initially and then recovers as the duration of the force increases. However, constant, or excessive force may lead to irreversible damage of the PDL, and even cause pulp necrosis and root resorption.115 Injury to the IAN and related neuropathy is rare during orthodontic treatment. However, the roots of molar or premolar teeth are situated in close proximity to the IAN, the IAN may be injured.116
Bioactive factors
Bioactive signaling factors secreted by bone lineage cells have the potential to modulate the physiological activity of the nerves. Osteoblastic cells express NGF and sema3a. The former is a nerve attractant molecule involved in nerve fiber maintenance and plasticity,117 and the latter is a repulsive molecule that inhibits fiber sprouting.118,119 The molecular network is disrupted after sympathectomy and the subsequent loss of VIP expression, leading to changes in the expressions of NGF and sema3a in rat mandible. As a result, CGRP-positive fibers invade the osteogenic layer due to the decrease in pro NGF and sema3a, and CGRP-positive fibers increase in the periosteum non-osteogenic layer due to an increase in mature NGF.70
Non-developmental diseases caused by reciprocal regulation between the nervous system and the stomatognathic system
Craniofacial diseases caused by neurological illnesses
Several main oral symptoms arise from the decline or loss of neurological function, such as facial paralysis, facial spasm, salivation, and Frey syndrome (Fig. 2).
Craniofacial diseases caused by neurological illnesses. a Facial paralysis. Lesions located between the cerebral cortex and the facial nerve nucleus lead to central facial paralysis. Extracranial lesions cause peripheral facial paralysis. b Facial spasm. Demyelination cause primary facial spasm and facial nerve injury may result in secondary facial spasm. c Salivation. Weakness or poor coordination of bulbar or facial muscles resulted from neurological diseases can cause salivation. d Frey syndrome. After parotid gland surgery, PSNS fibers can control sweat glands and blood vessels in the skin, leading to sweating and flushing during chewing. Created with BioRender.com
Facial paralysis
Facial paralysis is a typical neuro-stomatology disease that is divided into central facial paralysis and peripheral facial paralysis. Facial paralysis is caused by a dysfunction of the facial nerve, leading to the limitation of the activity of the facial muscles innervated by the nerve.120 Central facial paralysis lesions are located between the cerebral cortex and the facial nerve nucleus. Common etiologies include cerebrovascular diseases, intracranial tumor compression, brain trauma, and congenital facial nerve dysplasia.121,122,123 Symptoms of central facial paralysis manifest in facial muscle palsies below the opposite palpebral fissure, disappearance of the nasolabial fold, and food retention in the oral vestibule. Peripheral facial paralysis is more commonly caused by extracranial etiologies, including viral infections (especially herpes zoster virus),124 parotid malignant tumors, trauma, and even cold wind.125,126 Bell palsy is the most prevalent type of peripheral facial paralysis.127 Symptoms of Bell palsy include paralysis of all facial muscles on the lesion side, disappearance of forehead lines, inability to close the eyelids, sagging of the mouth angles, and even accompanying auditory changes and hypogeusia (Fig. 2a).128
Facial spasm
Facial spasm refers to involuntary convulsions or spasms129 of half of the facial muscles. It is classified as primary and secondary facial spasm.130 Primary facial spasm arises from demyelination caused by cerebellar pontine angle tumors131 and vascular malformations that compress the facial nerve root.132,133 This demyelination disrupts the normal flow of action currents along the nerve fiber, resulting in overexcitation of the facial nerve and subsequent facial spasm.134 Secondary facial spasm is caused by facial nerve injury due to facial paralysis, trauma, inflammation, and other factors.130 The twitching typically begins with the orbicularis oculi muscle and gradually extends to other facial expression muscles on the affected side.135 And the twitching of the angularis oris muscle is the most prominent symptom (Fig. 2b).129,136
Salivation
Saliva is secreted by salivary glands, which are stimulated by the PSNS, but the contraction of the salivary duct’s smooth muscle is controlled by the SNS. Therefore, neurological lesions can cause abnormal salivary secretion. The etiology of salivation may refer to weakness or poor coordination of bulbar or facial muscles, leading to poor lip seal, ineffective saliva control, and impaired swallowing mechanics.137 Therefore, neurological conditions like stroke, neuromuscular diseases like amyotrophic lateral sclerosis, and neurodegenerative diseases including PD, multiple system atrophy, and cerebral palsy can cause salivation.22 Excessive saliva accumulation in the mouth corner leads to a rapid propagation of microbes such as Candida albicans, Streptococcus spp, Staphylococcus spp, and herpesvirus, resulting in oral mucosal diseases, such as candidal stomatitis, coccal stomatitis, and herpes stomatitis (Fig. 2c).138,139,140
Frey syndrome
The salivary glands receive signals from the PSNS, while the sweat glands and cutaneous blood vessels are regulated by the SNS.141 Physiologically, saliva secretion and sweating are two separate processes. The salivary gland secretes saliva in response to chewing stimulation, while there is no significant change in the skin condition. However, after parotid gland surgery, PSNS fibers can control denervated sweat glands and blood vessels in the skin.142 Therefore, chewing can lead to not only saliva secretion from other salivary glands, but also sweating and flushing in the preauricular area due to increased PSNS activity. This phenomenon is known as Frey syndrome,143 which is characterized by sweating and flushing in response to mastication or a salivary stimulus.144 In fact, it is common symptom following salivary gland surgery.145 And other symptoms include face rash,146 burning, itching, forehead and scalp sweating147 and neuralgia (Fig. 2d).144
Neurological diseases caused by an aberrant stomatognathic system
While stomatognathic system abnormalities may not be the primary cause of neurological diseases, it is important to consider the potential links between them. Craniofacial symptoms or diseases, such as oral infection, OSCC, malocclusion and Sjogren syndrome (SS), can play a role in the development of neurological diseases. A comprehensive understanding of these links can aid in early prevention and treatment of these neurological diseases (Fig. 3).
Neurological diseases caused by an aberrant stomatognathic system. a The link between oral infection and CNS infection. Oral microbes are easy to invade the brain via hematogenous spread. b Perineural invasion resulting from tumors in the oral and maxillofacial regions. c Headache caused by malocclusion and sleep bruxism. d Sjogren syndrome. Mononuclear cells and lymphocytes invade lacrimal and salivary glands. Created with BioRender.com
The link between oral infection and CNS infection
The presence of abundant microflora in the oral cavity,148 combined with anatomical proximity of the brain and maxillofacial region, makes the CNS susceptible to infection. In analogy to the “gut-brain axis”, the proposed concept of the brain-oral axis suggests the profound influence of an oral microbiome on the brain.23,149 Hematogenous spread is the predominant mode of intracranial dissemination, and caries with periapical involvement and periodontitis are the most frequently-triggering factors.150 In addition, other oral and maxillofacial specific infections, including herpes simplex,151 herpes zoster, hand-foot-mouth disease,152 and oral tuberculosis,151 also invade the CNS along the peripheral nerve or blood-brain barrier, causing pain, meningitis or intracranial infection. Notably, even oral manipulations, like endodontic treatments, tooth extractions, oral surgery, and simple toothbrushing, may cause acute or chronic infection.153 When oral pathogens spread through the blood system or nerve fibers into the brain, severe consequences may occur, such as chronic inflammation, brain abscesses,150 ischemic stroke,154 neurodegenerative diseases, neuropsychological diseases,155 and even mortality. For instance, Porphyromonas gingivalis, a pivotal pathogen in gingivitis and periodontitis, can disrupt the blood-brain barrier via inflammation, which is a characteristic feature of cerebral small vessel disease,154 thereby increasing the risk of acute ischemic stroke (Fig. 3a).
Perineural invasion resulting from tumors in the oral and maxillofacial regions
Certain types of oral tumors, such as ACC and OSCC, can invade nerves, leading to PNI, which is characterized by tumor cells tracking along nerves and/or enveloping at least one-third of the nerve’s circumference.156 Furthermore, ACC is one of the most common salivary gland tumors, particularly in the small salivary glands of the palate and parotid gland. Due to its high propensity for spreading along nerves, ACC is capable of causing PNI in the head and neck region.157 Facial nerve invasion caused by ACC leads to facial paralysis, while invasion of the trigeminal nerve causes facial pain. Additionally, invasion of the glossopharyngeal nerve and hypoglossal nerve may result in tongue numbness and tongue movement disorders.24
The sixth most common malignant tumor, OSCC, can infiltrate the CNS via the facial and trigeminal nerves, leading to the development of intracranial space-occupying lesions24 and leptomeningeal disease.158 Although PNI in carcinoma of the lip is rare, malignant cells may trail along the IAN to the brainstem, resulting in leptomeningeal carcinomatosis.158 In addition, PNI appears in the advanced stages of tongue cancer.159 Patients may feel ear pain, throat pain, and pain in other areas involved in PNI.160 Although its mechanisms are not yet understood, PNI has been shown to be linked to an elevated risk of recurrence, regional transfer, distant metastasis, and overall worse prognosis (Fig. 3b).161
Aberrant stomatognathic system and headache
Headache is a prevalent condition that can be caused by various factors.162 Some studies have showed that malocclusion and sleep bruxism may contribute to the development of headache.163 Among different types of malocclusion, overbite, posterior crossbite, lingual crossbite, and lower crowding have been identified as potential risk factors for tension-type headaches in children and adolescents.162,164 The underlying mechanism may be related to the imbalanced bite, which can lead to tension in the masticatory muscles165,166 and subsequently trigger headache.167,168 Sleep bruxism, which is characterized by tooth grinding and jaw clenching during sleep,169 has also been associated with headache.163 This association may be due to the development of trigger points in the head and neck,170 which are hyperalgesic zones that can induce headache (Fig. 3c).171
Sjogren syndrome
Although the abnormal oral and maxillofacial system in SS is not the direct cause of neuropathy, neurological and oral symptoms often coexist in SS.172 SS is a chronic inflammatory autoimmune disease characterized by mononuclear lymphocytic infiltration in lacrimal and salivary glands,172,173 resulting in dry eyes and dry mouth. As the disease progresses, patients may experience various oral symptoms such as swallow dysfunction, oral malodour, rampant caries, periodontal disease, tongue papilla atrophy, sore tongue, salivary gland swelling or mumps, and poor denture retention.174 Additionally, orofacial myofunctional disorders and temporomandibular disorders (TMD) are common among SS patients,175 with main symptoms including orofacial pain and mandibular function limitation.176 In addition to orofacial regions, the nervous system is affected in SS, with CNS lesions such as aseptic meningitis,177 cerebellar syndromes178 and neuromyelitis optica and others, as well as peripheral neuropathy including sensory neuropathy, sensorimotor neuropathies, and cranial neuropathies.172 SS can even increase the risk of PD, dementia179 and depression (Fig. 3d).180
Interaction effects of neurological diseases and craniofacial diseases
The pathogeneses of some chronic diseases are exceedingly intricate, making it difficult to identify definitive instigating factors. In fact, in some cases, the diseases may mutually promote each other during their distinct stages. Consequently, this section aims to expound upon the plausible bidirectional associations between these diseases (Fig. 4).
Interaction effects of neurological and craniofacial diseases. a The interaction between neurodegenerative disorders and stomatognathic diseases. Periodontitis is conducive to the development of AD and PD, which increase the risk of stomatognathic diseases conversely. b Mutual promotion between psychic disorders and stomatognathic diseases. c The potential connection between abnormal mental state and temporomandibular disorders. d The link between pain and oral symptoms. Vascular compression leads to primary TN. And the etiology of secondary TN is various. No matter what kind of TN, it can cause a series of oral symptoms. P. gingivalis Porphyromonas gingivalis, LPS lipopolysaccharide, HPA hypothalamic-pituitary-adrenal. Created with BioRender.com
Neurodegenerative disorders
AD is the most common neurodegenerative disorder, its clinical characteristic is often manifested as progressive cognitive impairment.181 It has been discussed extensively that periodontitis is a risk factor for AD.182,183 Bacterial proteins and DNA from periodontal pathogens can provoke neuronal damage and cognitive impairment.184 Conversely, the severity of oral diseases is positively linked to AD,185 because patients in the advanced stage of AD lose intellectual and social abilities, as well as the ability to maintain proper oral hygiene practices. This leads to oral lesions like caries,186 periodontitis,187 stomatitis,188 ulcerations, angular cheilitis, candidiasis189,190 and oral dysfunction.191 The second most common neurodegenerative disorder, PD, is characterized by motor dysfunction.192 Periodontal inflammatory disease is also linked to the morbidity of PD.193 The pathogenic mechanism may involve neuroinflammation, which is a prevalent characteristic of various neurodegenerative disorders.155 Due to autonomic dysfunction, muscle stiffness, slowness of movement and tremor, PD patients are prone to developing stomatognathic diseases and motor impairments, like caries, periodontitis,194 TMJ dysfunction,195and oral dysfunctions (Fig. 4a).196,197,198
Psychological disorders
In addition to neurodegenerative diseases, there is mutual promotion between psychic disorders and stomatognathic diseases. Psychological factors, emotional stress, and schizophrenia may induce various oral diseases,199 such as oral ulcers, migratory stomatitis, polymorphous erythema, mucoid pemphigus, and chronic periodontitis.200,201,202 Among these psychological factors, the dyadic relation between depression and periodontal disease has been extensively studied.203 Depression is a relevant pathogenetic factor for periodontitis,25 and in turn, oral diseases can exacerbate the progression of depression (Fig. 4b).
Temporomandibular disorders
TMD are associated with an individual’s mental state. In fact, the biopsychosocial model of TMD was proposed long ago to describe how psychological distress,204 psychosocial impairment, and behavioral upset are highly prevalent among TMD patients.205,206,207 Stress and negative affect are considered potentially important risk factors for TMD.208 But the specific mechanism has not been fully clarified, which may refer to dysregulation of the hypothalamic-pituitary-adrenal209 and aberrant secretion of cortisol.210 However, the effect of TMD and associated pain on the nervous system is relatively weak. Patients with painful TMD have been found that salivary levels of NGF and brain-derived neurotrophic factor (BDNF) are lower compared to healthy control subjects211 NGF212 and BDNF213 are related to psychological impairment, which reflects a potential connection between an abnormal mental state and TMD. And patients suffering from painful TMD surely experience heightened self-perceived cognitive impairments and depressive symptoms.214 Furthermore, extensive alterations in brain structures have been observed in individuals afflicted with TMD pain,215 including modifications in the trigemino-thalamo-cortical system, the lateral and medial pain systems, periaqueductal gray-raphe magnus pathway and the motor system. Nevertheless, the relation between these neuropeptides and psychological distress is more complicated than previously thought, and further research is required to understand the intricate interaction between TMD and psychological distress (Fig. 4c).
Pain
Oral and maxillofacial pain is a significant issue that perplexes many patients and seriously impacts their facial muscle movement and daily routines. Pain-sensitive structures in the oral and maxillofacial region are distributed in the intracranial trigeminal and glossopharyngeal nerves, and in the extracranial oral and maxillofacial skin, subcutaneous tissue, muscle, TMJ, dental pulp, and oral mucosa.216 Therefore, diseases that stimulate pain-sensitive structures may cause oral and maxillofacial pain. The most common facial pain is trigeminal neuralgia (TN), which is divided into primary TN and second TN.26 Primary TN is typically caused by vascular compression with morphologic changes of the trigeminal nerve root.217 Second TN may be caused by an intracranial tumor,218 such as those in the cerebellopontine angle or multiple sclerosis, infiltrative malignant tumors, trauma, and rheumatologic diseases. Even extracranial infections can lead to TN, especially odontogenic infections, such as endodontic infections, and periodontal infections or abscesses.219 Acute pulpitis is a distinct form of dental inflammation that can elicit severe and spontaneous sharp pain upon compression of the involved nerve without timely drainage. Patients experience radiating pain along the second or third branch of the trigeminal nerve to the ipsilateral head, ear, face, and temporal region,220 often leading to secondary TN. Besides, herpes zoster infection can affect the trigeminal ganglion to trigger secondary TN.219 The underlying pathology of both primary TN and secondary TN is widely acceptable to be demyelination,218 which triggers impulses with high-frequency afterdischarges.221,222 Therefore, innocuous mechanical stimuli in the trigeminal territory, including light touch, cold air, brushing teeth, and eating, can trigger severe pain.217 As a result, patients may avoid basic hygiene practices, like washing their face, brushing their teeth, and smiling, leading to poor facial and oral hygiene accompanied by calculus and stomatitis. Furthermore, during the pain attack phase, patients may vigorously rub their facial skin to alleviate the pain, leading to partial abrasion and secondary infection.
Glossopharyngeal neuralgia (GPN) is a relatively rare condition that may be affected by both the nervous system and oral structure. Patients with GPN experience paroxysmal pain in the tonsils, pharynx, tongue base, and other areas. Similar to TN, one of the recognized lesions associated with GPN is nerve compression by a blood vessel at the root entry zone of the brainstem.223,224 Furthermore, CPN has also been linked to cerebellopontine angle masses, oropharyngeal tumors, multiple sclerosis, and TN.225,226,227 Also, GPN has trigger points that can elicit pain, such as swallowing, chewing, coughing, yawning, and talking. In addition to neuralgia, other symptoms may occur, such as excessive saliva, throat spasm,228 twitch, and epilepsy (Fig. 4d).229
Conclusions and future perspectives
This review summarizes the connection between neurodevelopment and craniofacial development, highlighting the intricate crosstalk between nerves and jawbones, as well as diseases among the two systems. The current research on the association between the nervous system and the stomatognathic system is extensive and intricate; however, it also has limitations. The underlying causes of congenital diseases in the stomatognathic system, such as Moebius syndrome, Parry–Romberg syndrome, and AS, remain unclear. Moreover, the connection between facial deformities and other neurodevelopmental disorders has not been established; this lack of understanding causes more complex disease management and higher costs, particularly without the aid of genetic screening. There is also a scarcity of studies that incorporate pathways related to the immune system and cation channels in jaw-regulating nerves. Research about the regulation of the CNS on the jawbone is also inadequate. At the molecular level, there is a lack of in-depth studies regarding the effect of acetylcholine and SP on the jawbone. In particular, the regulation of SP on the jawbone is perplexing, as opposing effects of SP have been observed at different concentrations. Interestingly, even at the same tested concentration, SP exerts different effects on the regulation of the jawbone. The role of SP may be strongly influenced by the specific surrounding environment, the duration of exposure, and the state of the jawbone. In addition, the interaction of neurological diseases and craniofacial diseases further complicates the issue, and the initial factors and the specific mechanism remain unclear.
Therefore, prioritizing neurodevelopment and neurological diseases related to the stomatognathic system is crucial for the timely prevention and treatment of oral diseases. It is imperative to investigate the contribution of published gene mutations to congenital diseases in both systems and expand the scope of gene mutation research. Such work would enhance the efficiency of prevention and treatment through embryo intervention and prenatal screening for dysplasia, as well as the early detection and diagnosis of refractory diseases, such as AD and TN. In addition, further investigations are necessary to examine the effects of bioactive factors, such as acetylcholine and SP, in regulating jawbone acquisition and loss. Furthermore, neural pathways mediated by the immune system and cation channels within jawbones are worth investigating. This may lead to the development of innovative strategies for neuro-bone tissue engineering.
Moreover, we found that the relationship between these two systems was far more complicated than what has been previously demonstrated. Based on existing research and obvious controversy, it is evident that the interaction mechanism between the nervous system and the stomatognathic system merits further investigation and potentially opens new research avenues.
Change history
11 September 2023
A Correction to this paper has been published: https://doi.org/10.1038/s41368-023-00250-3
References
Khan, M. T., Verma, S. K., Maheshwari, S., Zahid, S. N. & Chaudhary, P. K. Neuromuscular dentistry: Occlusal diseases and posture. J. Oral. Biol. Craniofac. Res. 3, 146–150 (2013).
Sandoval-Munoz, C. P. & Haidar, Z. S. Neuro-Muscular Dentistry: the "diamond" concept of electro-stimulation potential for stomato-gnathic and oro-dental conditions. Head. Face Med 17, 16 (2021).
Wang, G., Song, Z., Wang, J. & Qiu, C. Neuro-stomatology: an emerging inter-discipline worthy of attention. J. Chongqing Med. Univ. 46, 858–862 (2021).
Minoux, M. et al. Gene bivalency at Polycomb domains regulates cranial neural crest positional identity. Science 355, 11 (2017).
Bataille, C. et al. Different sympathetic pathways control the metabolism of distinct bone envelopes. Bone 50, 1162–1172 (2012).
Nowinski, W. L. 3D Atlas of the brain, Head and Neck in 2953 pieces. Neuroinformatics 15, 395–400 (2017).
Shoja, M. M. et al. Anastomoses between lower cranial and upper cervical nerves: a comprehensive review with potential significance during skull base and neck operations, Part II: glossopharyngeal, vagus, accessory, and hypoglossal nerves and cervical spinal nerves 1-4. Clin. Anat. 27, 131–144 (2014).
Moss, M. L. An introduction to the neurobiology of oro-facial growth. Acta Biotheor. 21, 236–259 (1972).
Dierssen, M. Down syndrome: the brain in trisomic mode. Nat. Rev. Neurosci. 13, 844–858 (2012).
Messiaen, L. et al. Clinical and mutational spectrum of neurofibromatosis type 1-like syndrome. JAMA J. Am. Med. Assoc. 302, 2111–2118 (2009).
Yamanaka, S. et al. Circulatory CNP rescues craniofacial hypoplasia in Achondroplasia. J. Dent. Res. 96, 1526–1534 (2017).
Lv, X., Gao, F. & Cao, X. Skeletal interoception in bone homeostasis and pain. Cell Metab. 34, 1914–1931 (2022).
Cooper, R. R. Nerves in cortical bone. Science 160, 327–328 (1968).
Hohmann, E. L., Elde, R. P., Rysavy, J. A., Einzig, S. & Gebhard, R. L. Innervation of periosteum and bone by sympathetic vasoactive intestinal peptide-containing nerve fibers. Science 232, 868–871 (1986).
Dimitri, P. & Rosen, C. The central nervous system and bone metabolism: an evolving story. Calcif. Tissue Int. 100, 476–485 (2017).
Elefteriou, F. Impact of the autonomic nervous system on the skeleton. Physiol. Rev. 98, 1083–1112 (2018).
Wan, Q. Q. et al. Crosstalk between bone and nerves within bone. Adv. Sci. 8, 24 (2021).
Fristad, I. Dental innervation: functions and plasticity after peripheral injury. Acta Odontol. Scand. 55, 236–254 (1997).
Kim, S. T. et al. Location of the mandibular canal and the topography of its neurovascular structures. J. Craniofac. Surg. 20, 936–939 (2009).
Renzi, G., Carboni, A., Perugini, M., Giovannetti, F. & Becelli, R. Posttraumatic trigeminal nerve impairment: a prospective analysis of recovery patterns in a series of 103 consecutive facial fractures. J. Oral. Maxillofac. Surg. 62, 1341–1346 (2004).
Tischfield, M. A. et al. Human TUBB3 mutations perturb microtubule dynamics, kinesin interactions, and axon guidance. Cell 140, 74–87 (2010).
Newall, A. R., Orser, R. & Hunt, M. The control of oral secretions in bulbar ALS/MND. J. Neurol. Sci. 139, 43–44 (1996).
Tonomura, S. et al. Intracerebral hemorrhage and deep microbleeds associated with cnm-positive Streptococcus mutans; a hospital cohort study. Sci. Rep. 6, 9 (2016).
Liu, X. H. et al. Perineural invasion in adenoid cystic carcinoma of the salivary glands: Where we are and where we need to go. Front. Oncol. 10, 10 (2020).
Saletu, A. et al. Controlled clinical and psychometric studies on the relation between periodontitis and depressive mood. J. Clin. Periodontol. 32, 1219–1225 (2005).
Harada, Y. et al. Cathepsin E in neutrophils contributes to the generation of neuropathic pain in experimental autoimmune encephalomyelitis. Pain 160, 2050–2062 (2019).
Labosky, P. A. & Kaestner, K. H. The winged helix transcription factor Hfh2 is expressed in neural crest and spinal cord during mouse development. Mech. Dev. 76, 185–190 (1998).
Southard-Smith, E. M., Kos, L. & Pavan, W. J. Sox10 mutation disrupts neural crest development in DOM Hirschsprung mouse model. Nat. Genet 18, 60–64 (1998).
Martik, M. L. & Bronner, M. E. Riding the crest to get a head: neural crest evolution in vertebrates. Nat. Rev. Neurosci. 22, 616–626 (2021).
Chai, Y. et al. Fate of the mammalian cranial neural crest during tooth and mandibular morphogenesis. Development 127, 1671–1679 (2000).
Kulesa, P. M., Bailey, C. M., Kasemeier-Kulesa, J. C. & McLennan, R. Cranial neural crest migration: new rules for an old road. Dev. Biol. 344, 543–554 (2010).
Gong, S. G. Cranial neural crest: migratory cell behavior and regulatory networks. Exp. Cell Res. 325, 90–95 (2014).
AlSarheed, M. A comparative study of oral health amongst trisomy 21 children living in Riyadh, Saudi Arabia: Part 1 caries, malocclusion, trauma. Saudi. Dent. J. 27, 220–223 (2015).
Hanookai, D., Nowzari, H., Contreras, A., Morrison, J. L. & Slots, J. Herpesviruses and periodontopathic bacteria in trisomy 21 periodontitis. J. Periodont. 71, 376–384 (2000).
Díaz-Quevedo, A. A., Castillo-Quispe, H. M. L., Atoche-Socola, K. J. & Arriola-Guillén, L. E. Evaluation of the craniofacial and oral characteristics of individuals with Down syndrome: a review of the literature. J. Stomatol Oral. Maxillofac. Surg. 122, 583–587 (2021).
Suri, S., Tompson, B. D. & Atenafu, E. Prevalence and patterns of permanent tooth agenesis in Down syndrome and their association with craniofacial morphology. Angle Orthod. 81, 260–269 (2011).
Cuoghi, O. A. et al. Prevalence of dental anomalies in permanent dentition of brazilian individuals with down syndrome. Open Dent. J. 10, 469–473 (2016).
Nuernberg, M. A. A. et al. Periodontal status of individuals with Down syndrome: sociodemographic, behavioural and family perception influence. J. Intellect. Disabil. Res. 63, 1181–1192 (2019).
Lugović-Mihić, L., Pilipović, K., Crnarić, I., Šitum, M. & Duvančić, T. Differential diagnosis of cheilitis - how to classify cheilitis? Acta Clin. Croat. 57, 342–351 (2018).
Lott, I. T. & Head, E. Dementia in Down syndrome: unique insights for Alzheimer disease research. Nat. Rev. Neurol. 15, 135–147 (2019).
Ulualp, S. Outcomes of tongue base reduction and lingual tonsillectomy for residual pediatric obstructive sleep apnea after adenotonsillectomy. Int Arch. Otorhinolaryngol. 23, e415–e421 (2019).
Lammert, M., Friedman, J. M., Kluwe, L. & Mautner, V. F. Prevalence of neurofibromatosis 1 in German children at elementary school enrollment. Arch. Dermatol. 141, 71–74 (2005).
Rasmussen, S. A. & Friedman, J. M. NF1 gene and neurofibromatosis 1. Am. J. Epidemiol. 151, 33–40 (2000).
Singhal, D. et al. Craniofacial neurofibromatosis: treatment of the midface deformity. J. Cranio MaxilloFac. Surg. 42, 595–600 (2014).
Cunha, K. S. G., Barboza, E. P., Dias, E. P. & Oliveira, F. M. Neurofibromatosis type I with periodontal manifestation. A case report and literature review. Br. Dent. J. 196, 457–460 (2004).
Ruggieri, M. et al. Unusual form of recurrent giant cell granuloma of the mandible and lower extremities in a patient with neurofibromatosis type 1. Oral. Surg. Oral. Med. Oral. Pathol. Oral. Radiol. Endod. 87, 67–72 (1999).
Friedrich, R. E., Giese, M., Schmelzle, R., Mautner, V. F. & Scheuer, H. A. Jaw malformations plus displacement and numerical aberrations of teeth in neurofibromatosis type 1: a descriptive analysis of 48 patients based on panoramic radiographs and oral findings. J. Cranio MaxilloFac. Surg. 31, 1–9 (2003).
Asgary, S. & Aminzadeh, N. Unilateral gingival enlargement in patient with neurofibromatosis type I. N. Y. State Dent. J. 78, 50–53 (2012).
Bongiorno, M. R., Pistone, G. & Arico, M. Manifestations of the tongue in neurofibromatosis type 1. Oral. Dis. 12, 125–129 (2006).
Curtin, J. P. & McCarthy, S. W. Perineural fibrous thickening within the dental pulp in type 1 neurofibromatosis - a case report. Oral. Surg. Oral. Med. Oral. Pathol. Oral. Radiol. Endod. 84, 400–403 (1997).
Takano, T. et al. The effect of parathyroid hormone (1-34) on cyclic AMP level, ornithine decarboxylase activity, and glycosaminoglycan synthesis of chondrocytes from mandibular condylar cartilage, nasal septal cartilage, and spheno-occipital synchondrosis in culture. J. Dent. Res. 66, 84–87 (1987).
Cohen, M. M. Jr Short-limb skeletal dysplasias and craniosynostosis: what do they have in common? Pediatr. Radiol. 27, 442–446 (1997).
Shiang, R. et al. Mutations in the transmembrane domain of FGFR3 cause the most common genetic form of dwarfism, achondroplasia. Cell 78, 335–342 (1994).
Matsushita, T. et al. FGFR3 promotes synchondrosis closure and fusion of ossification centers through the MAPK pathway. Hum. Mol. Genet. 18, 227–240 (2009).
Buchner, K. et al. Trigeminal stimulus menthol masks bitter off-flavor of artificial sweetener acesulfame-K. Foods 11, 12 (2022).
Blanton, P. L. & Jeske, A. H. The key to profound local anesthesia - neuroanatomy. J. Am. Dent. Assoc. 134, 753–760 (2003).
Ladizesky, M. G., Cutrera, R. A., Boggio, V., Mautalen, C. & Cardinali, D. P. Effect of unilateral superior cervical ganglionectomy on bone mineral content and density of rat’s mandible. J. Auton. Nerv. Syst. 78, 113–116 (2000).
Wu, Q. Q., Yang, B., Cao, C., Guang, M. K. & Gong, P. Age-dependent impact of inferior alveolar nerve transection on mandibular bone metabolism and the underlying mechanisms. J. Mol. Histol. 47, 579–586 (2016).
Yu, X. J. et al. Expression of neuropeptides and bone remodeling-related factors during periodontal tissue regeneration in denervated rats. J. Mol. Histol. 46, 195–203 (2015).
Wang, L. et al. Locally applied nerve growth factor enhances bone consolidation in a rabbit model of mandibular distraction osteogenesis. J. Orthop. Res. 24, 2238–2245 (2006).
Cherruau, M., Morvan, F. O., Schirar, A. & Saffar, J. L. Chemical sympathectomy-induced changes in TH-, VIP-, and CGRP-immunoreactive fibers in the rat mandible periosteum: Influence on bone resorption. J. Cell. Physiol. 194, 341–348 (2003).
Takeda, S. et al. Leptin regulates bone formation via the sympathetic nervous system. Cell 111, 305–317 (2002).
Li, Y. et al. Biodegradable magnesium combined with distraction osteogenesis synergistically stimulates bone tissue regeneration via CGRP-FAK-VEGF signaling axis. Biomaterials 275, 14 (2021).
Azuma, H., Kido, J., Ikedo, D., Kataoka, M. & Nagata, T. Substance P enhances the inhibition of osteoblastic cell differentiation induced by lipopolysaccharide from Porphyromonas gingivalis. J. Periodont. 75, 974–981 (2004).
Elefteriou, F., Campbell, P. & Ma, Y. Control of bone remodeling by the peripheral sympathetic nervous system. Calcif. Tissue Int. 94, 140–151 (2014).
Eimar, H., Tamimi, I., Murshed, M. & Tamimi, F. Cholinergic regulation of bone. J. Musculoskelet. Neuronal Interact. 13, 124–132 (2013).
Bajayo, A. et al. Skeletal parasympathetic innervation communicates central IL-1 signals regulating bone mass accrual. Proc. Natl Acad. Sci. USA 109, 15455–15460 (2012).
Khosla, S. Minireview: the OPG/RANKL/RANK system. Endocrinology 142, 5050–5055 (2001).
Kessler, J. A., Bell, W. O. & Black, I. B. Interactions between the sympathetic and sensory innervation of the iris. J. Neurosci. 3, 1301–1307 (1983).
Mauprivez, C. et al. Periosteum metabolism and nerve fiber positioning depend on interactions between osteoblasts and peripheral innervation in rat mandible. PloS one 10, e0140848 (2015).
Jiao, K. et al. β2-Adrenergic signal transduction plays a detrimental role in subchondral bone loss of temporomandibular joint in osteoarthritis. Sci. Rep. 5, https://doi.org/10.1038/srep12593 (2015).
Ribeiro, A. B. et al. Carotid sinus nerve stimulation attenuates alveolar bone loss and inflammation in experimental periodontitis. Sci. Rep. 10, 11 (2020).
Arredondo, J. et al. Muscarinic acetylcholine receptors regulating cell cycle progression are expressed in human gingival keratinocytes. J. Periodontal. Res. 38, 79–89 (2003).
Nguyen, V. T. et al. Choline acetyltransferase, acetylcholinesterase, and nicotinic acetylcholine receptors of human gingival and esophageal epithelia. J. Dent. Res. 79, 939–949 (2000).
Zoheir, N., Lappin, D. F. & Nile, C. J. Acetylcholine and the alpha 7 nicotinic receptor: a potential therapeutic target for the treatment of periodontal disease? Inflamm. Res. 61, 915–926 (2012).
Ordovas-Montanes, J. et al. The regulation of immunological processes by peripheral neurons in homeostasis and disease. Trends Immunol. 36, 578–604 (2015).
Procaccini, C., Pucino, V., De Rosa, V., Marone, G. & Matarese, G. Neuro-endocrine networks controlling immune system in health and disease. Front Immunol. 5, 143 (2014).
Li, C. H. & Amar, S. Morphometric, histomorphometric, and microcomputed tomographic analysis of periodontal inflammatory lesions in a murine model. J. Periodontol. 78, 1120–1128 (2007).
Yang, Y., Zhang, B., Yang, Y. F., Peng, B. B. & Ye, R. PLGA containing human adipose-derived stem cell-derived extracellular vesicles accelerates the repair of alveolar bone defects via transfer of CGRP. Oxid. Med. Cell. Longev. 2022, 14 (2022).
Wang, L. P. et al. Substance P stimulates bone marrow stromal cell osteogenic activity, osteoclast differentiation, and resorption activity in vitro. Bone 45, 309–320 (2009).
Wang, T. J. et al. Substance P incorporation in calcium phosphate cement for dental alveolar bone defect restoration. Mater. Sci. Eng. C. Mater. Biol. Appl. 69, 546–553 (2016).
Zhang, Y. B. et al. Local injection of substance P increases bony formation during mandibular distraction osteogenesis in rats. Br. J. Oral. Maxillofac. Surg. 52, 697–702 (2014).
Levi-Montalcini, R. The nerve growth factor 35 years later. Science 237, 1154–1162 (1987).
Wang, L. et al. Nerve growth factor and tyrosine kinase A in human salivary adenoid cystic carcinoma: expression patterns and effects on in vitro invasive behavior. J. Oral. Maxillofac. Surg. 64, 636–641 (2006).
Eppley, B. L., Snyders, R. V., Winkelmann, T. M. & Roufa, D. G. Efficacy of nerve growth factor in regeneration of the mandibular nerve: a preliminary report. J. Oral. Maxillofac. Surg. 49, 61–68 (1991).
Sicard, L. et al. Dental phenotype in Crouzon syndrome: a controlled radiographic study in 22 patients. Arch. Oral. Biol. 131, 105253 (2021).
Kyrkanides, S., Huang, H. & Faber, R. D. Neurologic regulation and orthodontic tooth movement. Front. Oral. Biol. 18, 64–74 (2016).
Beertsen, W., McCulloch, C. A. & Sodek, J. The periodontal ligament: a unique, multifunctional connective tissue. Periodontol 2000 13, 20–40 (1997).
Wang, K. et al. Axin2+ PDL cells directly contribute to new alveolar bone formation in response to orthodontic tension force. J. Dent. Res. 101, 695–703 (2022).
Watson, P. A. Function follows form: generation of intracellular signals by cell deformation. FASEB J. 5, 2013–2019 (1991).
Jiang, Y. et al. Mechanosensitive Piezo1 in periodontal ligament cells promotes alveolar bone remodeling during orthodontic tooth movement. Front. Physiol. 12, 767136 (2021).
Christensen, O. Mediation of cell volume regulation by Ca2+ influx through stretch-activated channels. Nature 330, 66–68 (1987).
Ei Hsu Hlaing, E., Ishihara, Y., Wang, Z., Odagaki, N. & Kamioka, H. Role of intracellular Ca(2+)-based mechanotransduction of human periodontal ligament fibroblasts. FASEB J. 33, 10409–10424 (2019).
Singh, I. J., Herskovits, M. S., Chiego, D. J. Jr. & Klein, R. M. Modulation of osteoblastic activity by sensory and autonomic innervation of bone. Prog. Clin. Biol. Res. 101, 535–551 (1982).
Heyeraas, K. J., Kvinnsland, I., Byers, M. R. & Jacobsen, E. B. Nerve fibers immunoreactive to protein gene product 9.5, calcitonin gene-related peptide, substance P, and neuropeptide Y in the dental pulp, periodontal ligament, and gingiva in cats. Acta Odontol. Scand. 51, 207–221 (1993).
Nishikawa, S. Systemic labeling and visualization of dental sensory nerves by the novel fluorescent marker AM1-43. Anat. Sci. Int. 81, 181–186 (2006).
Harris, J. A. Using c-fos as a neural marker of pain. Brain Res. Bull. 45, 1–8 (1998).
Fujiyoshi, Y., Yamashiro, T., Deguchi, T., Sugimoto, T. & Takano-Yamamoto, T. The difference in temporal distribution of c-Fos immunoreactive neurons between the medullary dorsal horn and the trigeminal subnucleus oralis in the rat following experimental tooth movement. Neurosci. Lett. 283, 205–208 (2000).
Novaes, A. P., da Rocha, M. J. & Leite-Panissi, C. R. Tooth movement activates the central amygdala and the lateral hypothalamus by the magnitude of the force applied. Angle Orthod. 80, 111–115 (2010).
Richardson, J. D. & Vasko, M. R. Cellular mechanisms of neurogenic inflammation. J. Pharm. Exp. Ther. 302, 839–845 (2002).
Maggi, C. A. Tachykinins and calcitonin gene-related peptide (CGRP) as co-transmitters released from peripheral endings of sensory nerves. Prog. Neurobiol. 45, 1–98 (1995).
O’Hara, A. H., Sampson, W. J., Dreyer, C. W., Pierce, A. M. & Ferguson, I. A. Immunohistochemical detection of nerve growth factor and its receptors in the rat periodontal ligament during tooth movement. Arch. Oral. Biol. 54, 871–878 (2009).
Vandevska-Radunovic, V., Kvinnsland, S. & Kvinnsland, I. H. Effect of experimental tooth movement on nerve fibres immunoreactive to calcitonin gene-related peptide, protein gene product 9.5, and blood vessel density and distribution in rats. Eur. J. Orthod. 19, 517–529 (1997).
Giannopoulou, C., Dudic, A. & Kiliaridis, S. Pain discomfort and crevicular fluid changes induced by orthodontic elastic separators in children. J. Pain. 7, 367–376 (2006).
Kondo, H. et al. Orthodontic tooth movement-activated sensory neurons contribute to enhancing osteoclast activity and tooth movement through sympathetic nervous signalling. Eur. J. Orthod. 44, 404–411 (2022).
Takiguchi, M. et al. Characteristics of mandibular canal branches related to nociceptive marker. J. Dent. Res. 100, 623–630 (2021).
Pramstraller, M., Schincaglia, G. P., Vecchiatini, R., Farina, R. & Trombelli, L. Alveolar ridge dimensions in mandibular posterior regions: a retrospective comparative study of dentate and edentulous sites using computerized tomography data. Surg. Radiol. Anat. 40, 1419–1428 (2018).
Wadu, S. G., Penhall, B. & Townsend, G. C. Morphological variability of the human inferior alveolar nerve. Clin. Anat. 10, 82–87 (1997).
Gangatharan, G., Schneider-Maunoury, S. & Breau, M. A. Role of mechanical cues in shaping neuronal morphology and connectivity. Biol. Cell. 110, 125–136 (2018).
Isomura, E. T. et al. Inferior alveolar nerve regeneration after bifocal distraction osteogenesis in dogs. J. Oral. Maxillofac. Surg. 71, 1810.e1–1811 (2013).
Day, I. N. & Thompson, R. J. Molecular cloning of cDNA coding for human PGP 9.5 protein. A novel cytoplasmic marker for neurones and neuroendocrine cells. FEBS Lett. 210, 157–160 (1987).
McCulloch, C. A., Lekic, P. & McKee, M. D. Role of physical forces in regulating the form and function of the periodontal ligament. Periodontology 24, 56–72 (2000).
Ren, Y., Maltha, J. C., Van ‘t Hof, M. A. & Kuijpers-Jagtman, A. M. Optimum force magnitude for orthodontic tooth movement: a mathematic model. Am. J. Orthod. Dentofac. Orthop. 125, 71–77 (2004).
Vandevska-Radunovic, V. Neural modulation of inflammatory reactions in dental tissues incident to orthodontic tooth movement. A review of the literature. Eur. J. Orthod. 21, 231–247 (1999).
Caviedes-Bucheli, J. et al. The effect of orthodontic forces on calcitonin gene-related peptide expression in human dental pulp. J. Endod. 37, 934–937 (2011).
Jadun, S., Miller, D. & Renton, T. Orthodontic-related nerve injuries: a review and case series. Br. Dent. J. 229, 244–248 (2020).
Aloe, L., Rocco, M. L., Bianchi, P. & Manni, L. Nerve growth factor: from the early discoveries to the potential clinical use. J. Transl. Med. 10, https://doi.org/10.1186/1479-5876-10-239 (2012).
Taniguchi, M. et al. Disruption of semaphorin III/D gene causes severe abnormality in peripheral nerve projection. Neuron 19, 519–530 (1997).
Gavazzi, I. Semaphorin-neuropilin-1 interactions in plasticity and regeneration of adult neurons. Cell Tissue Res 305, 275–284 (2001).
Li, J. et al. Modulation of the crosstalk between schwann cells and macrophages for nerve regeneration: a therapeutic strategy based on a multifunctional tetrahedral framework nucleic acids system. Adv. Mater. 34, https://doi.org/10.1002/adma.202202513 (2022).
Clouston, P. D., Sharpe, D. M., Corbett, A. J., Kos, S. & Kennedy, P. J. Perineural spread of cutaneous head and neck cancer. Its orbital and central neurologic complications. Arch. Neurol. 47, 73–77 (1990).
Lin, J. W., Chen, Y. C., Wen, H. M., Yang, Z. Y. & Zeng, J. S. Weakness of eye closure with central facial paralysis after unilateral hemispheric stroke predicts a worse outcome. J. Stroke Cerebrovasc. Dis. 26, 834–841 (2017).
Hoffmann, D. F., May, M. & Kubal, W. Slowly progressive facial paralysis due to vascular malformation of the brain stem. Am. J. Otol. 11, 357–359 (1990).
McCormick, D. P. Herpes-simplex virus as a cause of Bell’s palsy.1972. Rev. Med. Virol. 10, 285–289 (2000).
Peitersen, E. Bell’s palsy: the spontaneous course of 2,500 peripheral facial nerve palsies of different etiologies. Acta Oto-Laryngol. 122, 4–30 (2002).
Finsterer, J. Management of peripheral facial nerve palsy. Eur. Arch. Oto Rhino Laryn. 265, 743–752 (2008).
Hohman, M. H. & Hadlock, T. A. Etiology, diagnosis, and management of facial palsy: 2000 Patients at a facial nerve center. Laryngoscope 124, E283–E293 (2014).
Eviston, T. J., Croxson, G. R., Kennedy, P. G. E., Hadlock, T. & Krishnan, A. V. Bell’s palsy: aetiology, clinical features and multidisciplinary care. J. Neurol. Neurosurg. Psychiatry 86, 1356–1361 (2015).
Valls-Solé, J. Facial palsy, postparalytic facial syndrome, and hemifacial spasm. Mov. Disord. 17, S49–S52 (2002).
Yaltho, T. C. & Jankovic, J. The many faces of hemifacial spasm: differential diagnosis of unilateral facial spasms. Mov. Disord. 26, 1582–1592 (2011).
Arseni, C. & Petrovici, I. Persistent tonic facial spasm in brain stem tumours. J. Neurol. Sci. 7, 107–114 (1968).
Nielsen, V. K. Electrophysiology of the facial nerve in hemifacial spasm: ectopic/ephaptic excitation. Muscle Nerve 8, 545–555 (1985).
Luo, F. F., Xu, H., Zhang, M. & Wang, Y. Abnormal regional spontaneous brain activity and its indirect effect on spasm ratings in patients with hemifacial spasm. Front. Neurosci. 14, https://doi.org/10.3389/fnins.2020.601088 (2020).
Gutmann, L. AAEM minimonograph #37: facial and limb myokymia. Muscle Nerve 14, 1043–1049 (1991).
Wang, A. & Jankovic, J. Hemifacial spasm: clinical findings and treatment. Muscle Nerve 21, 1740–1747 (1998).
Hausser-Hauw, C., Roullet, E., Robert, R. & Marteau, R. Oculo-facio-skeletal myorhythmia as a cerebral complication of systemic Whipple’s disease. Mov. Disord. 3, 179–184 (1988).
Srivanitchapoom, P., Pandey, S. & Hallett, M. Drooling in Parkinson’s disease: a review. Parkinsonism Relat. Disord. 20, 1109–1118 (2014).
Kamilov, K. P., Kamalova, M. K. & Shokirova, F. A. Biology of mouth cavity in patients with chronic recurrent herpetic stomatitis. Uzbekiston Tibbiet Zh . 5, 5–11 (2018).
Donatsky, O. Cell-mediated and humoral immunity against oral streptococci, neisseria, staphylococci, and adult human oral mucosa antigens in recurrent aphthous stomatitis. Scand. J. Dent. Res. 86, 25–34 (1978).
Greenberg, M. S. Herpesvirus infections. Dent. Clin. North Am. 40, 359–368 (1996).
Hodges, G. J. & Johnson, J. M. Adrenergic control of the human cutaneous circulation. Appl. Physiol. Nutr. Metab. 34, 829–839 (2009).
Gardner, W. J. & McCubbin, J. W. Auriculotemporal syndrome; gustatory sweating due to misdirection of regenerated nerve fibers. J. Am. Med. Assoc. 160, 272–277 (1956).
Galli, S. Anatomic and functional bases of Frey’s syndrome: sensitive and sensorial stimulations. Rev. Laryngol. Oto. l Rhinol. 105, 89–91 (1984).
Motz, K. M. & Kim, Y. J. Auriculotemporal syndrome (Frey Syndrome). Otolaryngol. Clin. North Am. 49, 501–509 (2016).
Freedberg, A. S., Shaw, R. S. & McManus, M. J. The auriculotemporal syndrome. A clinical and pharmacologic study. J. Clin. Invest. 27, 669–676 (1948).
Palmeiro, A. G., Azurara, L., Pimentel, B. & Amaro, C. Case for diagnosis. A transient unilateral face rash upon eating: Frey syndrome. Bras. Dermatol. 98, 108–109 (2023).
Caliò, B., Wenning, G. K., Fanciulli, A. & Colosimo, C. Forehead and scalp gustatory sweating after temporomandibular joint surgery: an atypical presentation of Frey’s syndrome. Clin. Auton. Res. https://doi.org/10.1007/s10286-023-00931-3 (2023).
Dewhirst, F. E. et al. The human oral microbiome. J. Bacteriol. 192, 5002–5017 (2010).
Ihara, M. & Yamamoto, Y. Emerging evidence for pathogenesis of sporadic cerebral small vessel disease. Stroke 47, 554–560 (2016).
Moazzam, A. A., Rajagopal, S. M., Sedghizadeh, P. P., Zada, G. & Habibian, M. Intracranial bacterial infections of oral origin. J. Clin. Neurosci. 22, 800–806 (2015).
Nguyen, I., Urbanczyk, K., Mtui, E. & Li, S. Intracranial CNS infections: a literature review and radiology case studies. Semin. Ultrasound Ct. Mr. 41, 106–120 (2020).
Lee, T. C. et al. Diseases caused by enterovirus 71 infection. Pediatr. Infect. Dis. J. 28, 904–910 (2009).
Ewald, C., Kuhn, S. & Kalff, R. Pyogenic infections of the central nervous system secondary to dental affections-a report of six cases. Neurosurg. Rev. 29, 163–166 (2006). discussion 166-167.
Aarabi, G., Thomalla, G., Heydecke, G. & Seedorf, U. Chronic oral infection: an emerging risk factor of cerebral small vessel disease. Oral. Dis. 25, 710–719 (2019).
Hashioka, S. et al. The possible causal link of periodontitis to neuropsychiatric disorders: More than psychosocial mechanisms. Int. J. Mol. Sci. 20, https://doi.org/10.3390/ijms20153723 (2019).
Liebig, C., Ayala, G., Wilks, J. A., Berger, D. H. & Albo, D. Perineural invasion in cancer: a review of the literature. Cancer 115, 3379–3391 (2009).
Bjørndal, K. et al. Salivary gland carcinoma in Denmark 1990-2005: a national study of incidence, site and histology. Results of the Danish Head and Neck Cancer Group (DAHANCA). Oral. Oncol. 47, 677–682 (2011).
Sullivan, L. M. & Smee, R. Leptomeningeal carcinomatosis from perineural invasion of a lip squamous cell carcinoma. Australas. Radiol. 50, 262–266 (2006).
Sethi, S., Lu, M., Kapke, A., Benninger, M. S. & Worsham, M. J. Patient and tumor factors at diagnosis in a multi-ethnic primary head and neck squamous cell carcinoma cohort. J. Surg. Oncol. 99, 104–108 (2009).
Fahmy, M. D. et al. Are throat pain and otalgia predictive of perineural invasion in squamous cell carcinoma of the oropharynx? J. Oral. Maxillofac. Surg. 80, 363–371 (2022).
Rahima, B., Shingaki, S., Nagata, M. & Saito, C. Prognostic significance of perineural invasion in oral and oropharyngeal carcinoma. Oral. Surg. Oral. Med. Oral. Pathol. Oral. Radiol. Endod. 97, 423–431 (2004).
Komazaki, Y. et al. Association between malocclusion and headache among 12- to 15-year-old adolescents: a population-based study. Community Dent. Oral. Epidemiol. 42, 572–580 (2014).
De Luca Canto, G., Singh, V., Bigal, M. E., Major, P. W. & Flores-Mir, C. Association between tension-type headache and migraine with sleep bruxism: a systematic review. Headache 54, 1460–1469 (2014).
Lambourne, C., Lampasso, J., Buchanan, W. C. Jr., Dunford, R. & McCall, W. Malocclusion as a risk factor in the etiology of headaches in children and adolescents. Am. J. Orthod. Dentofac. Orthop. 132, 754–761 (2007).
Hinotsume, S. The difference from the point of view of masticatory function between normal occlusion and crowding, using Hellman’s dental stage. Shoni Shikagaku Zasshi 26, 535–555 (1988).
Hinotsume, S. et al. Occlusal development in children from the functional viewpoint. 4. Amount of masticatory muscle action in children with tooth crowding. Shoni Shikagaku Zasshi 24, 415–427 (1986).
Burnett, C. A., Fartash, L., Murray, B. & Lamey, P. J. Masseter and temporalis muscle EMG levels and bite force in migraineurs. Headache 40, 813–817 (2000).
Gonçalves, D. A. et al. Temporomandibular disorders are differentially associated with headache diagnoses: a controlled study. Clin. J. Pain. 27, 611–615 (2011).
Khoury, S., Carra, M. C., Huynh, N., Montplaisir, J. & Lavigne, G. J. Sleep bruxism-tooth grinding prevalence, characteristics and familial aggregation: a large cross-sectional survey and polysomnographic validation. Sleep 39, 2049–2056 (2016).
Molina, O. F., Peixoto, M. G., Eid, N. L. M., Aquilino, R. N. & Rank, R. C. I. C. Headache and bruxing behavior types in craniomandibular disorders (CMDs) patients. Rev. Neurocien. 19, 449–457 (2011).
Fernández-de-las-Peñas, C., Cuadrado, M. L., Arendt-Nielsen, L., Simons, D. G. & Pareja, J. A. Myofascial trigger points and sensitization: an updated pain model for tension-type headache. Cephalalgia 27, 383–393 (2007).
Margaretten, M. Neurologic manifestations of primary Sjögren syndrome. Rheum. Dis. Clin. North Am. 43, 519–529 (2017).
Sjögren, H. On knowledge of the keratoconjunctivitis sicca. VII. The sicca syndrome-an autoimmune disease. Acta Ophthalmol. 46, 201–206 (1968).
Hamburger, J. Orofacial manifestations in patients with inflammatory rheumatic diseases. Best. Pract. Res. Clin. Rheumatol. 30, 826–850 (2016).
Zanin, M. C., Garcia, D. M., Rocha, E. M. & de Felício, C. M. Orofacial motor functions and temporomandibular disorders in patients with Sjögren’s Syndrome. Arthritis Care Res. 72, 1057–1065 (2020).
Schiffman, E. et al. Diagnostic criteria for temporomandibular disorders (DC/TMD) for clinical and research applications: recommendations of the international RDC/TMD consortiumnetwork* and Orofacial Pain Special Interest Group†. J. Oral. Facial Pain. Headache 28, 6–27 (2014).
Rossi, R. & Valeria Saddi, M. Subacute aseptic meningitis as neurological manifestation of primary Sjögren’s syndrome. Clin. Neurol. Neurosurg. 108, 688–691 (2006).
Chen, Y. W. et al. Sjogren’s syndrome with acute cerebellar ataxia and massive lymphadenopathy: a case report. Acta Neurol. Taiwan. 22, 81–86 (2013).
Wang, Z. Z. et al. Risk of dementia or Parkinson’s disease in the presence of Sjögren’s syndrome: a systematic review and meta-analysis. Front. Integr. Neurosci. 16, https://doi.org/10.3389/fnint.2022.1027044 (2022).
Westhoff, G., Dörner, T. & Zink, A. Fatigue and depression predict physician visits and work disability in women with primary Sjögren’s syndrome: results from a cohort study. Rheumatology 51, 262–269 (2012).
2022 Alzheimer’s disease facts and figures. Alzheimers Dement. 18, 700–789 https://doi.org/10.1002/alz.12638 (2022).
Long, J. M. & Holtzman, D. M. Alzheimer disease: an update on pathobiology and treatment strategies. Cell 179, 312–339 (2019).
Chen, C. K., Wu, Y. T. & Chang, Y. C. Association between chronic periodontitis and the risk of Alzheimer’s disease: a retrospective, population-based, matched-cohort study. Alzheimers Res. Ther. 9, https://doi.org/10.1186/s13195-017-0282-6 (2017).
Dominy, S. S. et al. Porphyromonas gingivalis in Alzheimer’s disease brains: Evidence for disease causation and treatment with small-molecule inhibitors. Sci. Adv. 5, https://doi.org/10.1126/sciadv.aau3333 (2019).
Martande, S. S. et al. Periodontal health condition in patients with Alzheimer’s disease. Am. J. Alzheimers Dis. Other Demen. 29, 498–502 (2014).
Delwel, S. et al. Oral health and orofacial pain in older people with dementia: a systematic review with focus on dental hard tissues. Clin. Oral. Investig. 21, 17–32 (2017).
Maldonado, A., Laugisch, O., Bürgin, W., Sculean, A. & Eick, S. Clinical periodontal variables in patients with and without dementia-a systematic review and meta-analysis. Clin. Oral. Investig. 22, 2463–2474 (2018).
Gao, S. S., Chu, C. H. & Young, F. Y. F. Oral health and care for elderly people with Alzheimer’s disease. Int. J. Environ. Res. Public Health. 17, https://doi.org/10.3390/ijerph17165713 (2020).
Aragón, F. et al. Oral health in Alzheimer’s disease: a multicenter case-control study. Clin. Oral. Investig. 22, 3061–3070 (2018).
Delwel, S. et al. Oral hygiene and oral health in older people with dementia: a comprehensive review with focus on oral soft tissues. Clin. Oral. Investig. 22, 93–108 (2018).
Marchini, L., Ettinger, R., Caprio, T. & Jucan, A. Oral health care for patients with Alzheimer’s disease: an update. Spec. Care Dent. 39, 262–273 (2019).
Fonseca-Ornelas, L. et al. Parkinson-causing mutations in LRRK2 impair the physiological tetramerization of endogenous α-synuclein in human neurons. NPJ Parkinsons Dis. 8, https://doi.org/10.1038/s41531-022-00380-1 (2022).
Chen, C. K., Wu, Y. T. & Chang, Y. C. Periodontal inflammatory disease is associated with the risk of Parkinson’s disease: a population-based retrospective matched-cohort study. PeerJ. 5, https://doi.org/10.7717/peerj.3647 (2017).
Van Stiphout, M. A. E., Marinus, J., van Hilten, J. J., Lobbezoo, F. & de Baat, C. Oral health of Parkinson’s disease patients: a case-control study. Parkinsons Dis. 2018, https://doi.org/10.1155/2018/9315285 (2018).
Silva, P. F. et al. Impact in oral health and the prevalence of temporomandibular disorder in individuals with Parkinson’s disease. J. Phys. Ther. Sci. 27, 887–891 (2015).
Lobbezoo, F. & Naeije, M. Dental implications of some common movement disorders: a concise review. Arch. Oral. Biol. 52, 395–398 (2007).
Suttrup, I. & Warnecke, T. Dysphagia in Parkinson’s disease. Dysphagia 31, 24–32 (2016).
Ribeiro, G. R., Campos, C. H. & Rodrigues Garcia, R. C. M. Parkinson’s disease impairs masticatory function. Clin. Oral. Investig. 21, 1149–1156 (2017).
Shamim, T. The psychosomatic disorders pertaining to dental practice with revised working type classification. Korean J. Pain. 27, 16–22 (2014).
Gupta, O. P., Tiwarri, O. S., Salimeno, T. Jr. & Allen, D. R. Neuropsychiatric disorders and periodontal disease. Ann. Dent. 52, 28–33 (1993).
Monteiro da Silva, A. M., Oakley, D. A., Newman, H. N., Nohl, F. S. & Lloyd, H. M. Psychosocial factors and adult onset rapidly progressive periodontitis. J. Clin. Periodontol. 23, 789–794 (1996).
Moss, M. E. et al. Exploratory case-control analysis of psychosocial factors and adult periodontitis. J. Periodontol. 67, 1060–1069 (1996).
Dumitrescu, A. L. Depression and inflammatory periodontal disease considerations-An interdisciplinary approach. Front. Psychol. 7, https://doi.org/10.3389/fpsyg.2016.00347 (2016).
Dworkin, S. F. & LeResche, L. Research diagnostic criteria for temporomandibular disorders: review, criteria, examinations and specifications, critique. J. Craniomandib. Disord. 6, 301–355 (1992).
Lora, V. R., Canales Gde, L., Gonçalves, L. M., Meloto, C. B. & Barbosa, C. M. Prevalence of temporomandibular disorders in postmenopausal women and relationship with pain and HRT. Braz. Oral. Res. 30, https://doi.org/10.1590/1807-3107BOR-2016.vol30.0100 (2016).
Dworkin, S. F. et al. Reliability, validity, and clinical utility of the research diagnostic criteria for Temporomandibular Disorders Axis II Scales: depression, non-specific physical symptoms, and graded chronic pain. J. Orofac. Pain. 16, 207–220 (2002).
De La Torre Canales, G. et al. Prevalence of psychosocial impairment in temporomandibular disorder patients: a systematic review. J. Oral. Rehabil. 45, 881–889 (2018).
Fillingim, R. B. et al. Psychological factors associated with development of TMD: the OPPERA prospective cohort study. J. Pain. 14, T75–T90 (2013).
Staniszewski, K. et al. Temporomandibular disorders related to stress and HPA-axis regulation. Pain. Res Manag. 2018, 7020751 (2018).
Jo, K. B. et al. Association of pain intensity, pain-related disability, and depression with hypothalamus-pituitary-adrenal axis function in female patients with chronic temporomandibular disorders. Psychoneuroendocrinology 69, 106–115 (2016).
Jasim, H., Ghafouri, B., Gerdle, B., Hedenberg-Magnusson, B. & Ernberg, M. Altered levels of salivary and plasma pain related markers in temporomandibular disorders. J. Headache Pain. 21, https://doi.org/10.1186/s10194-020-01160-z (2020).
Chen, Y. W. et al. Significantly lower nerve growth factor levels in patients with major depressive disorder than in healthy subjects: a meta-analysis and systematic review. Neuropsychiatr. Dis. Treat. 11, 925–933 (2015).
Kishi, T., Yoshimura, R., Ikuta, T. & Iwata, N. Brain-derived neurotrophic factor and major depressive disorder: evidence from meta-analyses. Front. Psychiatry 8, 308 (2017).
Staniszewski, K., Ronold, E. H., Hammar, Å. & Rosén, A. Neurocognitive functioning in patients with painful temporomandibular disorders. J. Pain. Res 16, 2015–2025 (2023).
Yin, Y. et al. The neuro-pathophysiology of temporomandibular disorders-related pain: a systematic review of structural and functional MRI studies. J. Headache Pain. 21, 78 (2020).
Won, S. Y. et al. Neuroanastomosis and the innervation territory of the mental nerve. Clin. Anat. 27, 598–602 (2014).
Cruccu, G. et al. Trigeminal neuralgia: new classification and diagnostic grading for practice and research. Neurology 87, 220–228 (2016).
Cruccu, G., Di Stefano, G. & Truini, A. Trigeminal neuralgia. N. Engl. J. Med. 383, 754–762 (2020).
Olesen, J. Headache Classification Committee of the International Headache Society (IHS) The International Classification of Headache Disorders, 3rd edition. Cephalalgia 38, 1–211 (2018).
Siqueira, J. T. et al. Clinical study of patients with persistent orofacial pain. Arq. Neuropsiquiatr. 62, 988–996 (2004).
Burchiel, K. J. Abnormal impulse generation in focally demyelinated trigeminal roots. J. Neurosurg. 53, 674–683 (1980).
Waxman, S. G. & Brill, M. H. Conduction through demyelinated plaques in multiple sclerosis: computer simulations of facilitation by short internodes. J. Neurol. Neurosurg. Psychiatry 41, 408–416 (1978).
Teixeira, M. J., de Siqueira, S. R. & Bor-Seng-Shu, E. Glossopharyngeal neuralgia: neurosurgical treatment and differential diagnosis. Acta Neurochir. 150, 471–475 (2008).
Hamada, O. et al. A patient with vertebral artery dissection who initially suffered from pharyngeal pain. No Shinkei Geka 41, 1081–1085 (2013).
Nurmikko, T. J. Chapter 38 Trigeminal neuralgia and other facial neuralgias. Handb. Clin. Neurol. 81, 573–596 (2006).
Romero-Reyes, M. & Uyanik, J. M. Orofacial pain management: current perspectives. J. Pain. Res. 7, 99–115 (2014).
Kapnadak, S. G., Mikolaenko, I., Enfield, K., Gress, D. R. & Nathan, B. R. Ondine’s curse with accompanying trigeminal and glossopharyngeal neuralgia secondary to medullary telangiectasia. Neurocrit. Care 12, 395–399 (2010).
Honey, C. M. et al. Concurrent glossopharyngeal neuralgia and hemi-laryngopharyngeal spasm (HeLPS): a case report and a review of the literature. Neurosurgery 87, E573–E577 (2020).
Garretson, H. D. & Elvidge, A. R. Glossopharyngeal neuralgia with asystole and seizures. Arch. Neurol. 8, 26–31 (1963).
Boghosian-Sell, L. et al. Molecular mapping of the Edwards syndrome phenotype to two noncontiguous regions on chromosome 18. Am. J. Hum. Genet 55, 476–483 (1994).
Balasundaram, P. & Avulakunta, I. D. Edwards sy ndrome (StatPearls. Publishing, 2023).
Maheshwari, M. et al. PTPN11 mutations in Noonan syndrome type I: detection of recurrent mutations in exons 3 and 13. Hum. Mutat. 20, 298–304 (2002).
Tartaglia, M. et al. Mutations in PTPN11, encoding the protein tyrosine phosphatase SHP-2, cause Noonan syndrome. Nat. Genet 29, 465–468 (2001).
Tartaglia, M. et al. PTPN11 mutations in Noonan syndrome: molecular spectrum, genotype-phenotype correlation, and phenotypic heterogeneity. Am. J. Hum. Genet 70, 1555–1563 (2002).
Roberts, A. E., Allanson, J. E., Tartaglia, M. & Gelb, B. D. Noonan syndrome. Lancet 381, 333–342 (2013).
Pérez Jurado, L. A., Peoples, R., Kaplan, P., Hamel, B. C. & Francke, U. Molecular definition of the chromosome 7 deletion in Williams syndrome and parent-of-origin effects on growth. Am. J. Hum. Genet 59, 781–792 (1996).
Kozel, B. A. et al. Williams syndrome. Nat. Rev. Dis. Prim. 7, 42 (2021).
Butler, M. G. Prader-Willi syndrome: current understanding of cause and diagnosis. Am. J. Med Genet 35, 319–332 (1990).
Butler, M. G., Miller, J. L. & Forster, J. L. Prader-Willi syndrome - clinical genetics, diagnosis and treatment approaches: an update. Curr. Pediatr. Rev. 15, 207–244 (2019).
Bhattacharjee, K. et al. Crouzon syndrome and the eye: an overview. Indian J. Ophthalmol. 70, 2346–2354 (2022).
Kobayashi, Y., Ogura, K., Hikita, R., Tsuji, M. & Moriyama, K. Craniofacial, oral, and cervical morphological characteristics in Japanese patients with Apert syndrome or Crouzon syndrome. Eur. J. Orthod. 43, 36–44 (2021).
Tan, A. P. & Mankad, K. Apert syndrome: magnetic resonance imaging (MRI) of associated intracranial anomalies. Childs Nerv. Syst. 34, 205–216 (2018).
Yu, K., Herr, A. B., Waksman, G. & Ornitz, D. M. Loss of fibroblast growth factor receptor 2 ligand-binding specificity in Apert syndrome. Proc. Natl Acad. Sci. USA 97, 14536–14541 (2000).
White, S. M. et al. Growth, behavior, and clinical findings in 27 patients with Kabuki (Niikawa-Kuroki) Syndrome. Am. J. Med. Genet. A. 127A, 118–127 (2004).
Porntaveetus, T. et al. Expanding the oro-dental and mutational spectra of Kabuki Syndrome and expression of KMT2D and KDM6A in human tooth germs. Int. J. Biol. Sci. 14, 381–389 (2018).
Dentici, M. et al. Clinical spectrum of Kabuki-like syndrome caused by HNRNPK haploinsufficiency. Case report and literature review. Eur. J. Hum. Genet. 26, 477–477 (2018).
Miller, G. Neurological disorders - the mystery of the missing smile. Science 316, 826–827 (2007).
Verzijl, H., van der Zwaag, B., Cruysberg, J. R. M. & Padberg, G. W. Mobius syndrome redefined - a syndrome of rhombencephalic maldevelopment. Neurology 61, 327–333 (2003).
Lee, S. & Moon, C.-H. Orthodontic treatment in a patient with Moebius syndrome: a case report. Korean J. Orthod. 52, 451–460 (2022).
Picciolini, O. et al. Moebius syndrome: clinical features, diagnosis, management and early intervention. Ital. J. Pediatr. 42, 7 (2016).
Bucher, F., Fricke, J., Neugebauer, A., Cursiefen, C. & Heindl, L. M. Ophthalmological manifestations of Parry-Romberg syndrome. Surv. Ophthalmol. 61, 693–701 (2016).
Vaienti, L., Soresina, M. & Menozzi, A. Parascapular free flap and fat grafts: combined surgical methods in morphological restoration of hemifacial progressive atrophy. Plast. Reconstr. Surg. 116, 699–711 (2005).
Schultz, K. P., Dong, E., Truong, T. A. & Maricevich, R. S. Parry Romberg syndrome. Clin. Plast. Surg. 46, 231–237 (2019).
Tristani-Firouzi, M. et al. Functional and clinical characterization of KCNJ2 mutations associated with LQT7 (Andersen syndrome). J. Clin. Invest. 110, 381–388 (2002).
Andelfinger, G. et al. KCNJ2 mutation results in Andersen syndrome with sex-specific cardiac and skeletal muscle phenotypes. Am. J. Hum. Genet. 71, 663–668 (2002).
Sansone, V. & Tawil, R. Management and treatment of andersen-tawil syndrome (ATS). Neurotherapeutics 4, 233–237 (2007).
Elefteriou, F. et al. Leptin regulation of bone resorption by the sympathetic nervous system and CART. Nature 434, 514–520 (2005).
Inoue, H., Kondo, A. & Togari, A. Activation of the peripheral sympathetic nervous system increased the expression of cyclooxygenase-2 (COX-2) mRNA in mouse calvaria. Neurosci. Lett. 338, 37–40 (2003).
Rahman, S., Dobson, P. R. M., Bunning, R. A. D., Russell, R. G. G. & Brown, B. L. The regulation of connective tissue metabolism by vasoactive intestinal polypeptide. Regul. Pept. 37, 111–121 (1992).
Persson, E. & Lerner, U. H. The neuropeptide VIP potentiates IL-6 production induced by proinflammatory osteotropic cytokines in calvarial osteoblasts and the osteoblastic cell line MC3T3-E1. Biochem. Biophys. Res. Commun. 335, 705–711 (2005).
Mrak, E. et al. Calcitonin gene-related peptide (CGRP) inhibits apoptosis in human osteoblasts by β-catenin stabilization. J. Cell Physiol. 225, 701–708 (2010).
Acknowledgements
This work was supported by the Key Research and Development Program in Zhejiang Province (No. 2021C03059), the Funds of the Central Government Guiding Local Science and Technology Development (No. 2023ZY1060), and National Natural Science Foundation of China (No. 81801011).
Author information
Authors and Affiliations
Contributions
Y.W. and Y.L. conceived of the presented idea and drafted the manuscript. J.M and J.S. edited the manuscript and designed the figures. T.K. and Z.X. critically revised the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Wu, Y., Lan, Y., Mao, J. et al. The interaction between the nervous system and the stomatognathic system: from development to diseases. Int J Oral Sci 15, 34 (2023). https://doi.org/10.1038/s41368-023-00241-4
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41368-023-00241-4
This article is cited by
-
Publisher Correction: The interaction between the nervous system and the stomatognathic system: from development to diseases
International Journal of Oral Science (2023)