Abstract
Background and aims
Obesity predisposes to metabolic and cardiovascular diseases. Adipose tissue inflammation and systemic inflammation contribute to these complications. There are strong sex differences in adipose tissue distribution and in systemic inflammation. Women have more subcutaneous adipose tissue (SAT) and less visceral adipose tissue (VAT) than men. We explored the sex differences in the association between the different adipose compartments and inflammatory markers that are important in cardiometabolic disease pathophysiology.
Methods
Single-center observational cohort study with 302 individuals with a BMI ≥ 27 kg/m2. We were unable to acquire MRI data from seven individuals and from another 18 the MRI data were not usable, resulting in 277 people (155 men, 122 women), aged 55–81 years.
Intervention
We performed the following measurements: abdominal magnetic resonance imaging to measure VAT, and SAT (deep and superficial) volumes; circulating leukocyte counts and cytokine production capacity of peripheral blood mononuclear cells (PBMCs), circulating cytokines, adipokines, and targeted proteomics; abdominal sSAT biopsies for histology and gene expression.
Results
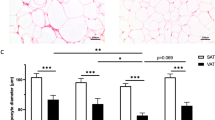
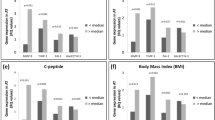
Only in women, (s)SAT volume was associated with circulating leukocytes, monocytes, and neutrophils. Circulating IL-6 and IL-18BP were associated with SAT volume in women and VAT in men. Several circulating proteins, including monocyte-colony-stimulating factor 1 and hepatocyte growth factor, are associated with sSAT in women and VAT in men. Only in women, SAT volume is associated with SAT expression of inflammatory proteins, including leptin, CD68, TNFα and IL-1α.
Conclusion
In women living with obesity, abdominal SAT volume, especially sSAT, is associated with circulating leukocytes and inflammatory proteins. In men, these parameters mainly show associations with VAT volume. This could be because only in women, sSAT volume is associated with sSAT expression of inflammatory proteins. These findings underscore that future research on adipose tissue in relation to cardiometabolic and cardiovascular disease should take sex differences into account.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
Data availability
The dataset used and/or analyzed during the current study is available from the corresponding author upon reasonable request.
References
Neeland IJ, Ayers CR, Rohatgi AK, Turer AT, Berry JD, Das SR, et al. Associations of visceral and abdominal subcutaneous adipose tissue with markers of cardiac and metabolic risk in obese adults. Obesity. 2013;21:E439–47.
Cancello R, Zulian A, Gentilini D, Maestrini S, Della Barba A, Invitti C, et al. Molecular and morphologic characterization of superficial- and deep-subcutaneous adipose tissue subdivisions in human obesity. Obesity. 2013;21:2562–70.
Brand T, Van Den Munckhof ICL, Van Der Graaf M, Schraa K, Dekker HM, Joosten LAB, et al. Superficial vs deep subcutaneous adipose tissue: sex-specific associations with hepatic steatosis and metabolic traits. J Clin Endocrinol Metab. 2021;106:e3881–9.
Cossins BC, van den Munckhof I, Rutten JHW, van der Graaf M, Stienstra R, Joosten LAB, et al. Sex-specific association between adipose tissue inflammation and vascular and metabolic complications of obesity. J Clin Endocrinol Metab. 2023;108:2537–49.
Bahrar H, Bekkering S, Stienstra R, Netea MG, Riksen NP. Innate immune memory in cardiometabolic disease. Cardiovasc Res. 2023; cvad030, https://doi.org/10.1093/cvr/cvad030.
Rohm TV, Meier DT, Olefsky JM, Donath MY. Inflammation in obesity, diabetes, and related disorders. Immunity. 2022;55:31–55.
Ter Horst R, van den Munckhof ICL, Schraa K, Aguirre-Gamboa R, Jaeger M, Smeekens SP, et al. Sex-specific regulation of inflammation and metabolic syndrome in obesity. Arterioscler Thromb Vasc Biol. 2020;40:1787–1800.
Pou KM, Massaro JM, Hoffmann U, Vasan RS, Maurovich-Horvat P, Larson MG, et al. Visceral and subcutaneous adipose tissue volumes are cross-sectionally related to markers of inflammation and oxidative stress: the Framingham Heart Study. Circulation. 2007;116:1234–41.
Fontana L, Eagon JC, Trujillo ME, Scherer PE, Klein S. Visceral fat adipokine secretion is associated with systemic inflammation in obese humans. Diabetes. 2007;56:1010–3.
Lee JJ, Pedley A, Hoffmann U, Massaro JM, Keaney JF, Vasan RS, et al. Cross‐sectional associations of computed tomography (CT)‐derived adipose tissue density and adipokines: the Framingham Heart Study. J Am Heart Assoc. 2016;5:e002545.
Koster A, Stenholm S, Alley DE, Kim LJ, Simonsick EM, Kanaya AM, et al. Body fat distribution and inflammation among obese older adults with and without metabolic syndrome. Obesity. 2010;18:2354–61.
Kim S-H, Chung J-H, Song S-W, Jung WS, Lee Y-A, Kim H-N. Relationship between deep subcutaneous abdominal adipose tissue and metabolic syndrome: a case control study. Diabetol Metab Syndr. 2016;8:10
Kurilshikov A, van den Munckhof ICL, Chen L, Bonder MJ, Schraa K, Rutten JHW, et al. Gut microbial associations to plasma metabolites linked to cardiovascular phenotypes and risk. Circ Res. 2019;124:1808–20.
Positano V, Gastaldelli A, Sironi AM, Santarelli MF, Lombardi M, Landini L. An accurate and robust method for unsupervised assessment of abdominal fat by MRI. J Magn Reson Imaging 2004;20:684–9.
Positano V, Cusi K, Santarelli MF, Sironi A, Petz R, Defronzo R, et al. Automatic correction of intensity inhomogeneities improves unsupervised assessment of abdominal fat by MRI. J Magn Reson Imaging. 2008;28:403–10.
Oosting M, Buffen K, Cheng SC, Verschueren IC, Koentgen F, van de Veerdonk FL, et al. Borrelia-induced cytokine production is mediated by spleen tyrosine kinase (Syk) but is Dectin-1 and Dectin-2 independent. Cytokine. 2015;76:465–472.
Koeken V, de Bree LCJ, Mourits VP, Moorlag SJ, Walk J, Cirovic B, et al. BCG vaccination in humans inhibits systemic inflammation in a sex-dependent manner. J Clin Invest. 2020;130:5591–5602.
Assarsson E, Lundberg M, Holmquist G, Bjorkesten J, Thorsen SB, Ekman D, et al. Homogenous 96-plex PEA immunoassay exhibiting high sensitivity, specificity, and excellent scalability. PLoS ONE. 2014;9:e95192.
Stienstra R, Duval C, Keshtkar S, van der Laak J, Kersten S, Muller M. Peroxisome proliferator-activated receptor gamma activation promotes infiltration of alternatively activated macrophages into adipose tissue. J Biol Chem. 2008;283:22620–7.
Cinti S, Mitchell G, Barbatelli G, Murano I, Ceresi E, Faloia E, et al. Adipocyte death defines macrophage localization and function in adipose tissue of obese mice and humans. J Lipid Res. 2005;46:2347–55.
Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc Ser B Methodol. 1995;57:289–300.
Swirski FK, Nahrendorf M. Leukocyte behavior in atherosclerosis, myocardial infarction, and heart failure. Science. 2013;339:161–6.
Netea MG, Joosten LA, Lewis E, Jensen DR, Voshol PJ, Kullberg BJ, et al. Deficiency of interleukin-18 in mice leads to hyperphagia, obesity and insulin resistance. Nat Med. 2006;12:650–6.
Bekkering S, van den Munckhof I, Nielen T, Lamfers E, Dinarello C, Rutten J, et al. Innate immune cell activation and epigenetic remodeling in symptomatic and asymptomatic atherosclerosis in humans in vivo. Atherosclerosis. 2016;254:228–36.
Shirai T, Nazarewicz RR, Wallis BB, Yanes RE, Watanabe R, Hilhorst M, et al. The glycolytic enzyme PKM2 bridges metabolic and inflammatory dysfunction in coronary artery disease. J Exp Med. 2016;213:337–54.
Lakoski SG, Cushman M, Criqui M, Rundek T, Blumenthal RS, D’Agostino RB Jr, et al. Gender and C-reactive protein: data from the Multiethnic Study of Atherosclerosis (MESA) cohort. Am Heart J. 2006;152:593–8.
Lee Y, Shin H, Vassy JL, Kim JT, Cho SI, Kang SM, et al. Comparison of regional body composition and its relation with cardiometabolic risk between BMI-matched young and old subjects. Atherosclerosis. 2012;224:258–65.
Saito T, Murata M, Otani T, Tamemoto H, Kawakami M, Ishikawa SE. Association of subcutaneous and visceral fat mass with serum concentrations of adipokines in subjects with type 2 diabetes mellitus. Endocr J. 2012;59:39–45.
Sam S, Haffner S, Davidson MH, D’Agostino RB Sr, Feinstein S, Kondos G, et al. Relation of abdominal fat depots to systemic markers of inflammation in type 2 diabetes. Diabetes Care. 2009;32:932–7.
Grundy SM, Adams-Huet B, Vega GL. Variable contributions of fat content and distribution to metabolic syndrome risk factors. Metab Syndr Relat Disord. 2008;6:281–8.
Monzon JR, Basile R, Heneghan S, Udupi V, Green A. Lipolysis in adipocytes isolated from deep and superficial subcutaneous adipose tissue. Obes Res. 2002;10:266–9.
Sniderman AD, Bhopal R, Prabhakaran D, Sarrafzadegan N, Tchernof A. Why might South Asians be so susceptible to central obesity and its atherogenic consequences? The adipose tissue overflow hypothesis. Int J Epidemiol. 2007;36:220–5.
Marinou K, Hodson L, Vasan SK, Fielding BA, Banerjee R, Brismar K, et al. Structural and functional properties of deep abdominal subcutaneous adipose tissue explain its association with insulin resistance and cardiovascular risk in men. Diabetes Care. 2014;37:821–9.
Rana MN, Neeland IJ. Adipose tissue inflammation and cardiovascular disease: an update. Curr Diab Rep. 2022;22:27–37.
Fox CS, Massaro JM, Hoffmann U, Pou KM, Maurovich-Horvat P, Liu CY, et al. Abdominal visceral and subcutaneous adipose tissue compartments: association with metabolic risk factors in the Framingham Heart Study. Circulation. 2007;116:39–48.
Snijder MB, Visser M, Dekker JM, Goodpaster BH, Harris TB, Kritchevsky SB, et al. Low subcutaneous thigh fat is a risk factor for unfavourable glucose and lipid levels, independently of high abdominal fat. The Health ABC Study. Diabetologia. 2005;48:301–8.
Agrawal S, Klarqvist MDR, Diamant N, Stanley TL, Ellinor PT, Mehta NN, et al. BMI-adjusted adipose tissue volumes exhibit depot-specific and divergent associations with cardiometabolic diseases. Nat Commun. 2023;14:266.
Alser M, Elrayess MA. From an apple to a pear: moving fat around for reversing insulin resistance. Int J Environ Res Public Health. 2022;19:14251.
Golan R, Shelef I, Rudich A, Gepner Y, Shemesh E, Chassidim Y, et al. Abdominal superficial subcutaneous fat: a putative distinct protective fat subdepot in type 2 diabetes. Diabetes Care. 2012;35:640–7.
Beasley LE, Koster A, Newman AB, Javaid MK, Ferrucci L, Kritchevsky SB, et al. Inflammation and race and gender differences in computerized tomography-measured adipose depots. Obesity. 2009;17:1062–9.
Cartier A, Côté M, Lemieux I, Pérusse L, Tremblay A, Bouchard C, et al. Sex differences in inflammatory markers: what is the contribution of visceral adiposity? Am J Clin Nutr. 2009;89:1307–14.
Feuerer M, Herrero L, Cipolletta D, Naaz A, Wong J, Nayer A, et al. Lean, but not obese, fat is enriched for a unique population of regulatory T cells that affect metabolic parameters. Nat Med. 2009;15:930–9.
Vasanthakumar A, Chisanga D, Blume J, Gloury R, Britt K, Henstridge DC, et al. Sex-specific adipose tissue imprinting of regulatory T cells. Nature. 2020;579:581–5.
Ryder E, Diez-Ewald M, Mosquera J, Fernández E, Pedreañez A, Vargas R, et al. Association of obesity with leukocyte count in obese individuals without metabolic syndrome. Diabetes Metab Syndr. 2014;8:197–204.
Yoshimura A, Ohnishi S, Orito C, Kawahara Y, Takasaki H, Takeda H, et al. Association of peripheral total and differential leukocyte counts with obesity-related complications in young adults. Obes Facts. 2015;8:1–16.
Nagareddy PR, Kraakman M, Masters SL, Stirzaker RA, Gorman DJ, Grant RW, et al. Adipose tissue macrophages promote myelopoiesis and monocytosis in obesity. Cell Metab. 2014;19:821–35.
Hino M, Inaba M, Goto H, Nishizawa Y, Tatsumi N, Nishino T, et al. Hepatocyte growth factor levels in bone marrow plasma of patients with leukaemia and its gene expression in leukaemic blast cells. Br J Cancer. 1996;73:119–23.
Bell LN, Ward JL, Degawa-Yamauchi M, Bovenkerk JE, Jones R, Cacucci BM, et al. Adipose tissue production of hepatocyte growth factor contributes to elevated serum HGF in obesity. Am J Physiol Endocrinol Metab. 2006;291:E843–8.
Osibogun O, Ogunmoroti O, Ferraro RA, Ndumele CE, Burke GL, Larson NB, et al. Favorable cardiovascular health is associated with lower hepatocyte growth factor levels in the multi-ethnic study of atherosclerosis. Front Cardiovasc Med. 2021;8:760281.
Levine JA, Jensen MD, Eberhardt NL, O’Brien T. Adipocyte macrophage colony-stimulating factor is a mediator of adipose tissue growth. J Clin Invest. 1998;101:1557–64.
Uhlén M, Fagerberg L, Hallström BM, Lindskog C, Oksvold P, Mardinoglu A, et al. Tissue-based map of the human proteome. Science. 2015. https://www.proteinatlas.org/ENSG00000173391-OLR1/single+cell+type.
Rasouli N, Yao-Borengasser A, Varma V, Spencer HJ, McGehee RE, Peterson CA, et al. Association of scavenger receptors in adipose tissue with insulin resistance in nondiabetic humans. Arterioscler Thromb Vasc Biol. 2009;29:1328–35.
Brinkley TE, Kume N, Mitsuoka H, Phares DA, Hagberg JM. Elevated soluble lectin-like oxidized LDL receptor-1 (sLOX-1) levels in obese postmenopausal women. Obesity. 2008;16:1454–6.
Stinson SE, Jonsson AE, Andersen MK, Lund MAV, Holm LA, Fonvig CE, et al. High plasma levels of soluble lectin‐like oxidized low‐density lipoprotein receptor‐1 are associated with inflammation and cardiometabolic risk profiles in pediatric overweight and obesity. J Am Heart Assoc. 2023;12:e8145.
Kataoka H, Kume N, Miyamoto S, Minami M, Moriwaki H, Murase T, et al. Expression of lectinlike oxidized low-density lipoprotein receptor-1 in human atherosclerotic lesions. Circulation. 1999;99:3110–7.
Markstad H, Edsfeldt A, Yao Mattison I, Bengtsson E, Singh P, Cavalera M, et al. High levels of soluble lectin like oxidized low‐density lipoprotein receptor‐1 are associated with carotid plaque inflammation and increased risk of ischemic stroke. J Am Heart Assoc. 2019;8:e009874.
Hofmann A, Brunssen C, Wolk S, Reeps C, Morawietz H. Soluble LOX‐1: a novel biomarker in patients with coronary artery disease, stroke, and acute aortic dissection? J Am Heart Assoc. 2020;9:e013803.
Kraler S, Wenzl FA, Georgiopoulos G, Obeid S, Liberale L, von Eckardstein A, et al. Soluble lectin-like oxidized low-density lipoprotein receptor-1 predicts premature death in acute coronary syndromes. Eur Heart J. 2022;43:1849–60.
Dregoesc MI, Ţigu AB, Bekkering S, van der Heijden C, Bolboacǎ SD, Joosten LAB, et al. Relation between plasma proteomics analysis and major adverse cardiovascular events in patients with stable coronary artery disease. Front Cardiovasc Med. 2022;9:731325.
Karason K, Girerd N, Andersson-Asssarsson J, Duarte K, Taube M, Svensson P-A, et al. Heart failure in obesity: insights from proteomics in patients treated with or without weight-loss surgery. Int J Obes. 2022;46:2088–94.
Koliaki C, Katsilambros N. Repositioning the role of tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) on the TRAIL to the development of diabetes mellitus: an update of experimental and clinical evidence. Int J Mol Sci. 2022;23:3225.
Acknowledgements
We thank all of the volunteers in the 300-OB cohort for their participation. LABJ, MGN, NPR and JHWR received a CVON grant from the Dutch Heart Foundation/Dutch Cardiovascular Alliance (IN CONTROL II: CVON2018-27). MGN is further supported by a European Research Council (ERC) Advanced Grant (FP/2007-2013/ERC grant 2012-322698), and a Spinoza Prize (NWO SPI 92-266). RS was supported by a VIDI-grant from the Dutch Research Council and a senior fellowship of the Dutch Diabetes Foundation. NPR was recipient of a grant of the ERA-CVD Joint Transnational Call 2018 supported by the Dutch Heart Foundation (JTC2018, project MEMORY; 2018T093).
Author information
Authors and Affiliations
Contributions
ICLM and HB: drafting of the manuscript, data acquisition, analysis, equal contribution. KS and TB: data acquisition. RH, MvdG, and HMD: data acquisition and analysis. RS, JG, LABJ, MGN, NPR, and JHWR: study design and funding, supervision of data acquisition and analyses. All co-authors contributed to writing of the manuscript. All authors provided critical revisions for important intellectual content, approved the final version submitted for publication, and agreed to be accountable for all aspects of the work.
Corresponding author
Ethics declarations
Competing interests
LABJ and MGN declare that they are scientific founders of Trained Therapeutics Discovery (TTxD) and LEMBA therapeutics. All other authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
van den Munckhof, I.C.L., Bahrar, H., Schraa, K. et al. Sex-specific association of visceral and subcutaneous adipose tissue volumes with systemic inflammation and innate immune cells in people living with obesity. Int J Obes 48, 523–532 (2024). https://doi.org/10.1038/s41366-023-01444-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41366-023-01444-9
This article is cited by
-
Preoperative risk factors associated with left ventricular dysfunction after bariatric surgery
Scientific Reports (2024)