Abstract
Introduction
Literature describing the impact of dietary intake on weight outcomes after bariatric surgery has not been synthesized. This study aimed to synthesize the evidence regarding any association between diet composition and weight outcomes post-bariatric surgery.
Methods
CINAHL, Cochrane, Embase, MEDLINE and Scopus were searched for adult studies up to June 2021 that assessed any association between dietary intakes (≥1-macronutrient, food group, or dietary pattern) and weight outcomes at 12-months or longer after bariatric surgery. Risk of bias and quality assessments were conducted using the Scottish Intercollegiate Guidelines Network checklists and the NHMRC’s Level of Evidence and Grades for Recommendations. Study findings were presented according to the time of post-surgery dietary intake assessment (≤12months, between 12 and 24 months, ≥24months).
Results
5923 articles were identified, 260 were retrieved for full text screening, and 36 were eligible for inclusion (9 interventional including five randomized-controlled trials, and 27 observational cohort studies; sample sizes: 20–1610; total sample: 5065; follow-up periods: 1 year–12 years; level of evidence: II to IV, risk of bias: low to high). Findings on the association between long-term weight outcomes and dietary composition up to 24-months were mixed. After 24-months, studies consistently suggested no significant associations between weight loss and macronutrient composition or core food group patterns, or between carbohydrate, protein or food group patterns and weight recurrence. A single cohort study reported a weak association between diet quality score and weight-recurrence after 24-months.
Conclusion
There was no strong evidence to support significant associations between diet composition and weight outcomes post-bariatric surgery. The heterogeneity in study design and quality may reduce generalizability to external populations. Individualized dietary recommendations may be useful to support long-term post-surgery weight outcomes. More studies are needed to define and measure diet quality in this patient cohort.
Registration
PROSPERO (CRD42021264120)
Similar content being viewed by others
Introduction
Bariatric surgery is considered the gold-standard treatment for inducing significant weight loss, which can alleviate obesity-related complications in people with severe obesity [1]. In recent years, the demand for bariatric surgery has increased with the rising prevalence of obesity [2]. From 2011 to 2019, the total number of bariatric surgeries performed worldwide rose from 158,000 to 256,000 [2]. However, weight non-response (insufficient weight loss) or weight recurrence (weight regain) are reported in as many as 1 in 2 patients at 2 years, and 3 in 5 patients at 12 years after surgery [3, 4]. These are associated with reduced quality of life, re-occurrence of obesity-related complications, deteriorated health, and ultimately, escalated health care costs and mortality [3,4,5,6,7].
Previous studies have explored the factors contributing to weight non-response or significant weight recurrence following bariatric surgery, and identified patients’ post-operative diet to be a key modifiable determinant of weight status post-surgery [5, 8]. In addition, Zarshenas and colleagues’ systematic review reported poor diet quality among patients at least one year after bariatric surgery [9]. These findings highlighted the role of dietary intakes in the management of weight after bariatric surgery, and the importance of nutritional interventions to improve the long-term diets of patients post-surgery.
At present, nutritional management guidelines for patients after bariatric surgery either focus on the diet texture progression within the first month post-operatively, or have based their overall diet recommendations, after texture progression (10–35% or >60 g/d protein, 30–70% or >130 g/d carbohydrates, 20–35% fats, and 5 serves of vegetables a day), on extrapolated evidence from non-surgical populations and/or small studies with weak evidence [10,11,12]. As bariatric surgery leads to significant changes in patients’ anatomy, physiology, and tolerance of specific foods and food volumes, dietary advice intended for the general population may not be suitable for patients post-bariatric surgery [13,14,15,16]. Depending on individual needs, stomach capacity, surgical outcomes and time after surgery, there may be a change in macronutrient requirements over-time. More evidence is needed to drive consensus and inform dietary recommendations for the medium to long-term post-surgery [9].
Over the past decade, there have been several studies examining the potential influence of dietary intake on weight loss and/or weight recurrence post-bariatric surgery. Therefore, the aim of this systematic review was to synthesize the available evidence regarding associations between post-surgery dietary intake (macronutrient composition and food patterns) and weight outcomes at least one year and longer after bariatric surgery.
Methods
The protocol for this review is registered in PROSPERO (CRD42021264120) and was conducted and reported as per the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) checklist [17].
Search strategy
A systematic search was conducted in electronic databases: CINAHL, Cochrane, Embase, MEDLINE and Scopus for human studies published in English language from all years up to and including June 2021. The basic search strategy was (post OR after OR following) “Bariatric surgery” OR “weight loss surgery” OR gastric bypass” OR “gastric sleeve” OR “sleeve gastrectomy” AND diet* OR nutrition* OR eat* OR macronutrient* OR ‘postoperative diet’. The full search strategy and subject headings used for each database are available in Supplementary Material 1.
Record screening and eligibility criteria
Title and abstract screening were conducted by HC. Full text screening was completed by members of the research team independently in groups of two (Group 1: HC and LR, Group 2: HC and ES, Group 3: HM and JM) using systematic review software, Rayyan (Rayyan Systems Inc., Cambridge) [18]. Any uncertainties around study inclusion were raised to the research team for discussion until consensus was reached. Eligible study designs were systematic reviews, meta-analyses, interventional and cohort studies of adults (18 years of age or above) without pre-existing life-threatening conditions who received bariatric surgery (any type). Outcomes included at least one post-surgery dietary variable (reported at least 1 macronutrient, food group or dietary pattern) that was compared to any post-surgery weight outcome(s). Studies were included if their analyses involved: (1) weight outcomes in response to a prescribed diet; (2) a comparison of weight change between participant groups and their concurrent diet; and/or (3) correlation analysis of association between weight outcomes and diet. Studies were excluded if: (i) any participants were pregnant or breastfeeding (ii) the participants were inpatients, (iii)a post-surgery progression diet (i.e. texture modified or less than 1 month post-surgery), (iv) measures of adherence to a specific diet or diet preferences without indicating the exact diet being followed, or (v) if intake analysis were limited to energy, micronutrients, test meals, single meals, or supplements only.
Data extraction
Data extraction was performed by HC and cross-checked by LR. Information extracted included country of publication, participant characteristics including pre- and post-surgery health parameters, assessment timelines, dietary assessment method, dietary intakes (energy, macronutrients, food/dietary patterns) and weight outcomes at any timepoint: excess weight loss, odds of reaching >50% excess weight loss, total weight loss, average monthly weight loss, initial weight loss, BMI loss, risk of obesity remission i.e. BMI < 30 kg/m2, presence of weight recurrence as defined by the study authors as exceeding a nominated percentage of weight gain after nadir weight (lowest weight post-surgery), odds of weight recurrence, risk of weight recurrence. Key findings were those regarding any association between dietary intake variables and weight outcome(s), and/or comparisons between intervention and control groups or groups of participants achieving/not achieving pre-defined weight outcome(s) and dietary intake. Statistical analysis results reported in studies were extracted, including correlation co-efficient, odds ratio, hazard ratio and 95% Confidence Interval and interpreted according to conventional standards established by Cohen [19].
Quality assessment
Individual studies were matched to the Australian National Health and Medical Research Council (NHMRC) levels of evidence and grades for recommendation guidelines depending on study design [20]. Studies were first assigned a level based on the potential of the study design to adequately answer the defined research question(s): with level I being the highest level of evidence, followed by II, III-1, III-2, III-3, and IV (lowest) [20]. The Scottish Intercollegiate Guidelines Network (SIGN) Risk of Bias checklists for cohort, case-control and controlled trial study designs were then used to determine the risk of bias (low risk, acceptable risk, and high risk) [21]. The level of evidence and risk of bias of each study were assessed by HC and cross-checked by LR independently with blinding.
Data synthesis
To synthesize reporting differences between studies, dietary and outcome data were treated as follows: Intake assessments reported over different timeframes were grouped and presented as three main post-surgery time-dependent categories: up to one year (≤12 months); between one and two years ( > 12 to <24-months); and two years or longer (≥24 months). Studies were then further categorised within each assessment timeframe according to the dietary variables reported (macronutrient composition or food pattern). Within these dietary categories, all associated weight outcomes were included regardless of follow-up timeframes (equivalent or longer than the dietary timeframes) and grouped as two main outcome categories: (1) weight loss (including excess weight loss (EWL), initial weight loss (IWL), total weight loss (TWL), body mass index (BMI) loss), and obesity remission (i.e. reaching a BMI of <30 kg/m2); or (2) weight recurrence measures of odds ratio, hazard ratio, or presence of weight recurrence from nadir weight that had exceeded a study-specified percentage.
Data analysis
The bodies of evidence regarding the associations between post-surgery weight outcomes and individual macronutrients and food patterns were assessed and graded using the NHMRC Guidelines [20]. In accordance with these guidelines, the bodies of evidence were assessed based on five components, and each component has been graded based on a set of standard criteria [20].
Recommendations were then deduced from these bodies of evidence and graded based on the combined gradings from each graded component. The possible grades for recommendations were: Grade A (body of evidence can be trusted to guide practice); Grade B (body of evidence can be trusted to guide practice in most situations); Grade C (body of evidence provides some support for recommendation(s) but care should be taken in its application); and Grade D (body of evidence is weak, and recommendation must be applied with caution). The grading of evidence was conducted by HC, cross-checked by LR, then reviewed and achieved consensus with ES, JM, AB, and CL.
Results
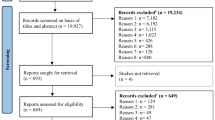
The screening process of this review is outlined in Fig. 1. A total of 5923 records were identified and title/abstract screened after the removal of 1495 duplicates. A total of 260 records were retrieved for full text screening, and 36 papers were included in this review. Reasons for exclusion were listed in Fig. 1.
Study characteristics
The key characteristics of the 36 included studies are summarized in Table 1 [22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57]. The retrieved study designs included nine intervention studies (5 randomised controlled trials, 3 non-randomized controlled trials, 1 pre-post-intervention) [29, 32, 34, 36, 37, 47, 52, 53, 55]. Interventions delivered typically consisted of lifestyle modifications with or without a prescribed diet plan, where participants’ dietary intakes (macronutrient composition and/or food group pattern) were recorded and compared against pre-intervention values and/or between study intervention or control groups. In total, 27 observational cohort studies (8 prospective and 19 retrospective) were included [22,23,24,25,26,27,28, 30, 31, 33, 35, 38,39,40,41,42,43,44,45,46, 48,49,50,51, 54, 56, 57]. These studies compared the diets of participants grouped according to their weight status, and/or conducted direct tests of association between dietary variable(s) and weight outcome(s).
A range of bariatric surgery procedures were reported among the included studies, with some individual studies reporting more than one type. The most common procedures were Roux en Y gastric bypass (RYGB) (N = 22 studies) [24, 25, 28, 30,31,32,33, 36, 37, 39, 40, 43,44,45,46, 48,49,50,51,52, 56, 57] and sleeve gastrectomy (SG) (N = 13 studies) [22, 25,26,27, 34,35,36, 40, 43, 46, 51, 53, 54]. Others included laparoscopic adjustable gastric banding (LAGB) (N = 4 studies) [29, 34, 52, 55], vertical banded gastroplasty (VBG) (N = 3 studies) [38, 41, 47], gastric bypass (did not specify type) (N = 3 studies) [23, 38, 41], gastric banding (did not specify type) (N = 1 study) [38], and anastomosis gastric bypass (OAGB) (N = 1 study) [25]. One study did not specify the type of bariatric surgery involved [42]. Dietary intake assessment tools (from most to least common) were food records (3–7 days), followed by 24-hour recalls, food frequency questionnaires, and other lifestyle or behavior surveys collecting dietary data as part of the tool [22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57]. Sample size ranged from 20 to 1610 participants (total N = 5065) with drop-out rate from 0 to 61%. The reported mean age of participants ranged from 32.7 ± 1.6 years to 57.3 ± 8.7 years. The proportion of female participants totaled across all studies was 75% (excluding the single study where gender ratio was not reported) [54]. The majority of studies did not mention the proportion of participants with insulin resistance or type 2 diabetes mellitus (T2DM). Even though some studies did report on the percentage of participants with at least one co-morbidity, they did not specify the exact proportion of participants with T2DM [33, 57]. Among studies that reported on them, the rate of participants with insulin resistance or T2DM mellitus ranged from 11.6%-52.5% [22, 25, 35, 36, 40]. Timepoint of measurement or assessment of post-surgery diets ranged from 6 months to 12 years. Significant post-surgery weight outcomes were reported in all studies from 1 to 12 years follow up.
Quality assessment
The level of evidence and risk of bias outcomes are reported in Table 1. There were no Level I studies, nine Level II studies [24, 37, 38, 43, 47,48,49, 52, 53], 22 Level III studies [22, 23, 25,26,27,28,29, 31, 33,34,35,36, 39, 40, 42, 44, 46, 50, 51, 54,55,56], and five Level IV studies [30, 32, 41, 45, 57]. Risk of bias assessment deemed 13 studies to be low risk [22, 23, 25, 28, 35, 38,39,40,41, 43, 48,49,50], 14 studies were acceptable risk [24, 26, 27, 29, 30, 33, 34, 36, 37, 44,45,46, 51, 57] and nine studies were high risk studies [31, 32, 42, 47, 52,53,54,55,56]. Studies’ level of evidence and risk of bias were both taken into consideration during quality assessment.
Study findings
Table 2 summarises the findings from individual studies, of which, 27 studies compared the concurrent diets of study groups (per intervention status or weight outcome) and 16 studies conducted direct tests of association between dietary variable(s) and weight outcome(s). The types of diet being assessed, key findings, and limitations for each included study. Study findings are presented according to the timeframe when dietary intakes were measured post-surgery ≤12-months, between 12 and 24 months, and ≥24 months.
Diet ≤12-months post-surgery and weight loss
Sixteen studies described diets up to 12-month and observed weight losses up to ten-years [24, 25, 27, 29, 30, 34, 36, 38,39,40, 47,48,49, 52,53,54]. Regarding carbohydrate, significant inverse associations with weight losses were supported by three observational cohort studies (total N = 1888, up to 10-years follow-up) [30, 38, 40]. However, one RCT (prescribed protein enriched, low-carbohydrate diet vs prescribed normal protein and carbohydrate diet, with high reported adherence) and four observational cohort studies showed no significant associations (total N = 368, up to 8-years follow-up) [24, 25, 39, 48, 53]. Regarding protein, significant positive associations with weight loss was supported by five observational cohort studies (total N = 2232, up to 10-years follow-up) [30, 38, 40, 48, 49]. On the contrary, one RCT (prescribed protein enriched, low-carbohydrate diet vs prescribed normal protein- and carbohydrate diet, with high reported adherence), one pseudo-RCT (prescribed protein-enriched diet vs no prescribed diets, with poor reported adherence), and four observational cohort studies did not report significant associations with weight loss (total N = 444, up to 8-years follow-up) [24, 25, 27, 29, 39, 53]. Regarding fat, significant inverse associations with weight losses were supported by two observational cohort studies (total N = 1799, up to 10-years follow-up) [38, 40]. However, five observational cohort studies did not report any significant associations (total N = 400, up to 8-years follow-up). A single RCT that involved a lifestyle intervention did not result in any significant between-group differences in macronutrient intakes nor weight changes[52].
Food pattern, specifically fruit and vegetable intakes, were reported in three studies (one RCT, two non-randomized controlled trials) [34, 36, 47]. All studies reported significantly greater weight losses in intervention groups compared with controls after lifestyle interventions [34, 36, 47]. However, no significant differences in fruit and vegetable intakes were noted between intervention and control groups within the single RCT [47]. Although between-group intake differences were not measured within the two non-randomized controlled trials, both studies reported significant increases in fruit and vegetable intakes in their intervention groups when compared to pre-intervention intakes [34, 36]. As both studies included a physical activity component, it was not possible to attribute outcomes to diet alone [34, 36]. There was inadequate information provided on other food patterns including dairy, meat and grains, as they were not measured in the single RCT [47], whereas the two non-randomized controlled trials reported no significant changes in their intakes compared to pre-intervention [34, 36]. Lastly, a single cohort study observed the effect of varying purine contents of the diet (exact food group intakes not reported), which did not show any significant association with post-surgery weight [54].
Diet ≤12-months post-surgery and weight recurrence
No studies examined weight recurrence during this period, presumably due to the short timeframe to enable observation of weight recurrence.
Diet between 12- and 24-months post-surgery and weight loss
Six studies described diets between 12- and 24-months post-surgery and observed weight losses up to five years post-surgery [31, 37, 41, 51, 52, 55]. Diet was also described and compared with weight recurrence at two years post-surgery by a single cohort study [42]. Regarding carbohydrate, significant inverse associations with weight losses was supported by one observational cohort study (total N = 75, with up to 2-years follow-up), whereas a lack of association was suggested by one non-randomized controlled trial and one observational cohort study (total N = 290, with up to 5-years follow-up). Additionally, Lindroos et al. assessed the potential impact of different types of carbohydrate intakes and reported greater weight loss for participants with higher intakes of mono- or di-saccharides and lower intakes of polysaccharides, though the potential impacts of total carbohydrate intake was not assessed [41]. Findings for protein included one observational cohort study (N = 75, with up to 2-years follow-up) suggesting positive associations [31], one observational cohort study (N = 375, with up to 2-years follow-up) suggesting inverse associations [41], and two studies (one non-randomized controlled trial and one observational cohort) suggesting no significant associations with weight loss (total N = 290, with up to 5-years follow-up) [51, 55]. Regarding fat, only one observational cohort study (N = 375, up to two-years) [41] supported positive associations with weight loss whereas three studies (one non-randomized controlled trial, two observational cohorts) reported having no significant associations (total N = 365, up to 5-years) [31, 51, 55]. A single RCT that involved a lifestyle intervention did not result in any significant between-group differences in macronutrient intakes nor weight outcomes [52]. The relationship between post-surgery weight loss and food pattern was assessed by a single RCT [37], providing monthly home delivered meals with a personalized menu plan of 4 serves vegetables, 2–4 serves meat, and 1–2 serves grains per day. The intervention resulted in greater weight loss than a control group with no delivered meals or prescribed diet and may indicate some benefits for similar types of intervention or prescribed menu plans [37].
Diet between 12- and 24-months post-surgery and weight recurrence
Macronutrient composition, food pattern, and weight recurrence at up to 18-months post-surgery were described by a cohort study [42]. The findings of this study favored a diet with daily intakes of 3–5 fat, fruit, and vegetable exchanges for less weight recurrence, though the portion size of each food exchange was not reported [42].
Diet at ≥24-months post-surgery and weight loss
Fourteen studies described dietary intakes after 24-months post-surgery and weight loss up to 12 years post-surgery [23, 26, 32, 33, 35, 39, 43,44,45, 47, 50, 51, 56, 57]. The absence of association was supported by 11 out of 11 observational cohort studies regarding carbohydrate and fat intakes (Total N = 1305, with up to 12-years follow-up) [23, 26, 33, 35, 39, 43,44,45, 50, 51, 56], and by 12 out of 12 observational cohort studies for protein intake (Total N = 1402, with up to 12-years follow-up) [23, 26, 33, 35, 39, 43,44,45, 50, 51, 56, 57]. A pre-/post-interventional study of individuals who had experienced weight recurrence at 3 years post-surgery reported significant weight loss from a three-month diet with 45% carbohydrate, 35% protein, and 20% fat [32]. Despite high adherence, the researchers did not compare to pre-intervention intakes, and an incentivized physical activity component made attribution of results to diet alone impossible [32]. Regarding food pattern, the absence of association between weight loss and any core food group, except fruit and vegetables, was supported by 2 out of 2 observational cohort studies (total N = 147, with up to 5 years follow-up) [23, 33]. A positive association between weight loss and fruit and vegetables was suggested by one RCT, where a lifestyle intervention resulted in significantly greater weight loss and concurrent significantly higher fruit and vegetable intakes in the intervention group compared to controls (N = 30, with up to 3 years follow-up) [47]. However, the component of physical activity prevented the outcomes being attributed to diet alone [47]. Despite this, three cohort studies reported no associations between fruit or vegetable intakes with weight loss up to 9-years (total N = 244, with up to 9-years follow-up) [23, 33, 57].
Diet at ≥24-months post-surgery and weight recurrence
Eight observational cohort studies described dietary intakes after 24-months and observed weight recurrence up to 9 years [22, 23, 26, 28, 33, 35, 46, 57]. For macronutrient intake composition, the lack of association between weight recurrence with carbohydrate and protein intakes was largely consistent across studies: Carbohydrate (6 out of 6 cohort studies, total N = 410, with up to five-years follow-up [22, 23, 26, 28, 33, 46]); Protein (5 [22, 23, 28, 33, 57] out of 6 cohort studies [22, 23, 26, 28, 33, 57], total N = 366 out of 403, with up to 9 years follow-up). The findings regarding fat intakes were mixed, though the absence of association was supported by 4 [22, 23, 28, 33] out of 6 cohort studies [22, 23, 26, 28, 33, 35] (total N = 269 out of 392, with up to 7-years follow-up [22, 23, 28, 33]). Differences in the definition of significant weight recurrence across studies (i.e. >2% to >25% recurrence of weight [22, 23, 26, 28, 33, 35]) may partially account for the mixed results. Notably, in the cohort study with the longest follow-up (7-years) and strictest criteria for Group 1 (>50% excess weight loss at first year and maintaining <25% weight recurrence until 7-years), higher fat intakes, in addition to total energy intake, were linked to a 4-fold increased risk of weight recurrence within a cox hazard regression model. Group 1 participants also reported significantly lower intakes of fat than Group 2 participants (>25% weight recurrence at 7-years).
Regarding food patterns, a lack of association between weight recurrence and any core food group was reported by 4 out of 4 observational cohort studies (total N = 326, with total follow-up of 8.9 years) [23, 28, 33, 47], except for fruit, where 1 out of the 4 studies was suggestive of an inverse association between fruit intakes and weight recurrence [28]. Diet quality score, measured using the Brazilian version of the Healthy Eating Index, was assessed by one cohort study that showed a weak inverse association with the odds of weight recurrence with a higher diet quality score (achieved by having a balanced daily servings of grains, vegetables, fruits, beans, meats, dairy products, fats and oils, sugar and sweets, restricted intakes of saturated fats and cholesterol, and having a high variety of foods within the diet) [28].
Grading of evidence and recommendations
Tables 3 to 6 provide a summary of the results assessed using the NHMRC body of evidence framework [20].
The body of evidence for diet ≤12 months post-surgery and weight loss is summarized in Table 3. Overall, the body of evidence was inconsistent regarding the presence or absence of any positive or negative associations between weight loss and macronutrient composition. While the findings for food group patterns appeared to be consistent, co-variables related to study design prevented the effect from being attributed to dietary intake alone. Therefore, no recommendations could be drawn.
The body of evidence for diet between 12- and 24-months post-surgery and weight loss and weight recurrence is summarized in Table 4. The body of evidence was inconsistent regarding the presence or absence of any associations between weight loss and diet, and there was inadequate information to draw conclusions on whether, and in what way, dietary intakes were associated with weight recurrence. Therefore, no recommendations could be drawn.
The body of evidence for diet ≥24 months post-surgery and weight loss is summarized in Table 5. The overall body of evidence was consistent for the absence of any significant associations between weight loss and all macronutrient intakes and food patterns. This body of evidence contributed to the recommendation that long-term diets post-bariatric surgery can be individualized with flexibility as to macronutrient and food pattern composition (Grade B –the body of evidence can be trusted to guide practice in most situations).
The body of evidence for diet ≥24 months post-surgery and weight recurrence is summarized in Table 6. The body of evidence was consistent for the absence of any significant associations between weight recurrence and carbohydrate, protein, and food patterns. However, the study findings for the association between weight recurrence and fat intakes was inconsistent, and an inverse association with diet quality was reported by just one cohort study. Therefore, two recommendations can be made: long-term diets post-bariatric surgery can be individualized with flexibility to macronutrient and food group composition due to lack of association (Grade C); and that a high diet quality can be encouraged in long-term post-surgery diets for reduction of weight recurrence (Grade D).
Study findings for the dietary components not able to be graded
It was not within the scope of this review to have an in-depth analysis of the associations between energy intake and weight outcomes, due to not being included in the search term for systematic retrieval, and food patterns beyond core food group intakes due to not being consistently reported (e.g. specific food intakes like sandwiches, packaged foods or sweets that were defined differently across studies). These findings are presented as part of Table 2.
Discussion
To our knowledge, the current review is the first to systematically synthesize existing literature reporting on associations between macronutrient composition, food patterns and weight outcomes post-bariatric surgery. Our review found that current evidence is related to the assessment of diet in distinct timeframes: ≤12 months, between 12- and 24-months and ≥24 months after surgery. Relationships between macronutrient intake and weight loss up to 24 months were inconclusive due to inconsistent findings between several studies of varying quality. Very few studies reported on food patterns, where the lack of studies and poor study design also contributed to inconclusive evidence. However, at 24 months and longer the evidence was consistent across several study findings for no association between macronutrient intake or food group pattern and weight loss, and between carbohydrate, protein and food group pattern and weight recurrence. An inverse association between weight recurrence and diet quality was reported from a single cohort study. Therefore, the existing body of evidence, overall, does not support a specific macronutrient composition or food pattern for optimal weight outcomes after bariatric surgery.
Previous research has suggested or attempted to establish the best macronutrient composition to support post-surgery weight loss [9, 40]. However, our review found the evidence base for associations with macronutrient composition and food pattern relied on less robust study designs and many contained confounding factors resulting in mixed results over different timeframes. The interpretation of association, therefore, was limited due to the lack of high-quality study designs. Despite this, the most notable phenomenon was that any relationship between macronutrients, food group pattern and weight outcomes, seemed to have lost significance after 24-months post-surgery, as studies that assessed dietary intakes after 24-months post-surgery found no associations whilst there was a mix of studies reporting the presence or absence of associations before 24 months [22, 23, 26, 28, 30, 31, 33, 35, 38, 39, 41,42,43,44,45,46, 50, 51, 55,56,57]. More well designed RCTs or prospective cohort studies are required to explore the potential associations between post-surgery weight outcomes with short- to medium- term dietary intakes (<24-months).
Although studies reporting on energy intake post-surgery were not systematically searched as part of this review, an inverse association with weight loss and positive association with weight recurrence was largely supported by those included studies that assessed energy intake [22,23,24,25,26, 28, 30, 31, 33, 35, 38,39,40,41, 43,44,45,46, 50, 51, 56]. Previous studies have debated the roles of long-term dietary restriction and food malabsorption in post-bariatric surgery weight outcomes [58,59,60,61,62,63,64]. However, there are known contributing factors that make it difficult to ascribe weight outcomes solely to energy intake, for example, the known prevalence of under-reporting dietary intakes among this population [59, 62, 63] and/or differences in energy balance as a result of individual basal requirements and physical activity levels [62, 65]. In Novais et al., only those with >50% excess weight loss reported an energy intake that was significantly lower than their estimated energy requirements. Furthermore, Benson-Davies et al. [59]. found participants who maintained their weight loss achieved a 2100-kilojoule deficit in energy balance (through a combination of lower energy intake and higher step counts) when compared to participants who experienced weight recurrence. Similarly, Forbush et al. suggested energy expenditure, rather than energy intake alone, to be a predictor of weight loss [62]. Given the limited reporting of physical activity levels in this population [65], energy balance may be an important focus for future research and practice to support long-term weight loss/maintenance post-bariatric surgery.
For many, the ultimate goal of achieving and sustaining weight losses after bariatric surgery is to improve the management of obesity-related complications. With the modest direct associations between post-surgical weight and diet composition found in this review, it may be sensible to place higher emphasis on exploring the impacts of diet composition on non-weight parameters of health, and specifically, those that are associated with the management of obesity-related complications. In the current review, studies that have reported non-weight clinical parameters mostly focused on the associations between body composition, quality of life and protein intakes [29, 37, 49, 53, 55], due to protein’s role in muscle mass maintenance, believed to be beneficial for weight loss maintenance. However, the overall evidence from the current review is mixed and does not support a high-protein diet for weight loss outcomes long-term post-surgery. This finding aligns with those of two previous systematic reviews focusing on the associations of protein intakes and body composition, which resulted in inconclusive findings [66, 67]. Larger and higher quality studies that place greater emphasis on obesity-related health parameters, in addition to weight, are required to explore how post-surgery diets may influence these parameters. Also, a recent study found no significant correlations between the extent of post-surgery weight loss and improvements in cardiovascular risk factors [68]. While this information was from a single study, it is apparent that future studies would benefit from placing additional emphasis on the relationship between post-surgery dietary intakes and clinical parameters, such as cardiometabolic health, in patients with or without weight non-response or recurrence at long-term post-bariatric surgery.
With the need to explore the relationships between obesity-related health parameters beyond weight, and in the absence of associations between weight non-response or recurrence with individual macronutrients and food groups, focusing on the overall quality of individuals’ diets may provide a more holistic approach to improving patients’ post-surgery eating pattern. In this review, Da Silva et al. was the only study retrieved that assessed diet quality in a systemic manner using an established tool, and suggested an ongoing weak association with weight re-occurrence after two years post-surgery [28]. Other identified studies that mentioned participants’ diet quality either did not use any established tool to measure diet quality [23, 33, 42], or did not adequately report participants’ dietary intakes [69,70,71,72,73], thus preventing comparisons across studies and, therefore, not included in the review as part of the data analyses for diet quality [23, 33, 42] or excluded for not meeting eligibility criteria [69,70,71,72,73]. Additionally, it is not known how useful or valid are established diet quality tools for use in this population [74, 75]. At present, existing research undertaken to derive and validate diet quality indices does not specifically consider bariatric surgery population [28, 70,71,72,73,74,75,76]. The potential changes in physiology and/or dietary needs after bariatric surgery may reduce the generalizability of diet quality indices from the general population to the bariatric surgery population. Without an accepted definition or measurement of high diet quality following bariatric surgery, it is not possible to compare the potential influence of diet quality on different obesity-related health parameters between studies and over-time. Future studies are needed to establish a consensus or criterion for the definition of a high-quality diet after bariatric surgery and to develop and validate a bariatric-specific diet quality measurement tool.
The differences in surgical procedure are often speculated as a contributor to different weight outcomes. Only one included study compared outcomes between different surgical types (SG versus RYGB), with no significant differences observed for weight outcomes and macronutrient composition [43]. Although findings for LAGB studies may not be generalizable across different surgery types, as they did not involve the resection of the gastro-intestinal tract, only 4 out of the 36 included studies involved post-LAGB patients. However, the differences in dietary intakes across surgical procedures were not the main interest of the current review. Since most of the included studies in this review reported single procedures only, and key findings did not differ across single or mixed surgeries or different surgery types, separate reporting of findings per surgery type was not attempted by this review. More studies may be needed to assess whether differences in surgical procedure can moderate the association between diet and post-surgery weight, and if so, whether such differences are significant enough to warrant separate dietary advice per surgery type. Another commonly speculated contributor to different weight outcomes is concomitant medications that may influence body weight (e.g. anti-depressants, corticoids, insulin) and the presence of T2DM, which may result in less weight loss than participants without T2DM [77]. In our review, only four of the included studies excluded participants taking these medications, but it was unclear whether the type(s) of medications considered in each study were the same [22, 23, 27, 28, 37]. Similarly, the rate of participants with insulin resistance or T2DM were only reported in a few studies and was often combined with other co-morbidities for assessment of the effects of number of co-morbidities on post-surgery weight [22, 25, 35, 36, 40]. The single study that assessed the potential impact of T2DM on excess weight loss reported no significant associations [25]. Another study reported higher rate of weight recurrence among those with insulin resistance, but acknowledged participants’ higher body fat percentage or BMI may have contributed in part to the results [22]. Future studies may need to clarify whether participants with medications or conditions that may influence participants’ weight are included in their statistical analysis, in order for higher confidence that the recorded weight changes are attributable to participants’ lifestyle.
The strengths of this review include the emphasis on long-term outcomes after bariatric surgery, and being the first review to systematically examine any potential associations between weight and dietary intakes after bariatric surgery. Limitations include the lack of inclusion of energy intake as a search criterion and the inability to discriminate the results by the type of surgery. The application, interpretation and generalization of results required subjective judgements to arrive at final recommendations. The retrieved studies were limited by a generally poor evidence base and poor consistency due to their methodological flaws and heterogeneity in study design, including different units of measure and different methods of assessment of association. Most studies did not account for dietary changes made before or after their dietary assessments, which may have played a role in weight status measured at time of study. Furthermore, the definitions used for post-surgery weight outcomes (weight non-response or weight recurrence) lacked standardization. These limitations align with the findings of a recent review that called for a research-derived definition of clinically significant post-surgery weight recurrence [78]. This lack of standardization limited comparisons between studies and the grading of evidence to support specific recommendations, particularly those assessing short-term dietary intakes (up to 24 months). The unknown contributors to weight outcomes such as physical activity, genetics and individual motivation, especially in studies with longer durations, were poorly reported within the included studies. Although the treatment of known confounders by the study authors were taken into consideration during risk of bias assessment, their interference on the potential clinical impact of the synthesized evidence was inevitable. Generalizability and applicability of studies to other bariatric contexts was also affected by individual study designs that may have incurred risk of selection bias, or contained inadequate information (e.g. adherence to prescribed diets, or dietary intakes prior to study intervention), to enable reproducibility of study outcomes to external populations. Moreover, the retrieved studies had a high proportion of female participants. Lastly, the results were prone to inherent limitations of dietary assessment methodologies such as recall bias and under-reporting. While there are standardized methods for excluding implausible reporting [79], only two studies in our review attempted to address the known risk of under-reporting by comparing two methods of self-report (a 72 h recall versus a three day food record) to evaluate compliance to prescribed diets, which reported high compliance and similar energy intakes [53, 54]. The remaining studies simply acknowledged this limitation, but did not identify nor exclude potential under-reporters [24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52, 55,56,57]. Even though one study hoped to use the Goldberg cut-offs to assess implausible intakes among their participants within 12-months post-surgery, said method assumes weight stability and was deemed unsuitable for use for patients who had yet to reach weight maintenance stage after surgery [29]. Importantly, there are no standardized energy intake cut-offs for this population to assist with assessment of implausible intakes, which further complicated the exclusion of implausible reporters. As this limitation was discussed by some of the included studies as a potential contributor to the lack of association found between dietary intakes and weight outcomes, future studies on patients post-bariatric surgery may benefit from including the assessment of implausible intakes using estimated energy requirements, supplementing food diaries with weighed food records or photographed pictures of participants’ meals, adjusting for total energy intake based on two methods (e.g. a food frequency questionnaire and three 24 h food recalls), or other standardized methods, to help improve accuracy of dietary assessments.
Conclusion
In view of the overall finding that there is a lack of evidence to support the strong association of any particular diet composition or pattern with weight loss or recurrence, it is not unreasonable to suggest that long-term dietary advice post-bariatric surgery can be individualized with flexibility as to the macronutrient and food composition, or that a focus on diet quality may be beneficial for the reduction of weight recurrence over the longer term. However, these recommendations should be taken with care until higher quality studies further confirm and strengthen these statements. Well-designed prospective trials with standardized reporting and monitoring of dietary intakes including energy intake/expenditure, coupled with the considerations of potential moderators of weight, such as the type of surgery, may be beneficial to help clarify the relationships between weight outcomes and dietary intakes.
Data availability
Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study. The search strategy of the current review is included as Supplementary Material 1.
References
Torgersen Z, Osmolak A, Forse RA. Sleeve gastrectomy and Roux En Y gastric bypass: current state of metabolic surgery. Curr Opin Endocrinol Diabetes Obes. 2014;21:352–7.
Estimate of Bariatric Surgery Numbers, 2011-2019. In: American Society for Metabolic and Bariatric Surgery. 2021. https://asmbs.org/resources/estimate-of-bariatric-surgery-numbers.
Velapati SR, Shah M, Kuchkuntla AR, Abu-Dayyeh B, Grothe K, Hurt RT, et al. Weight recurrence after bariatric surgery: prevalence, etiology, and treatment. Curr Nutr Rep. 2018;7:329–34.
Magro DO, Geloneze B, Delfini R, Pareja BC, Callejas F, Pareja JC. Long-term weight recurrence after gastric bypass: a 5-year prospective study. Obes Surg. 2008;18:648–51.
Karmali S, Brar B, Shi X, Sharma AM, De Gara C, Birch DW. Weight recidivism post-bariatric surgery: a systematic review. Obes Surg. 2013;23:1922–33.
Lupoli R, Lembo E, Saldalamacchia G, Avola CK, Angrisani L, Capaldo B. Bariatric surgery and long-term nutritional issues. World J. Diabetes. 2017;8:464–74.
Kim J, Waitzman N, Simper S, McKinlay R, Cottam D, Surve A, et al. Effects of post-operative nutritional disorders following bariatric surgery on health care cost and use. Obes Surg. 2021;31:2503–10.
Jessri M, Kaouk L, Hsu AT, Tanuseputro P. Modifiable factors associated with weight recurrence after bariatric surgery: a scoping review. F1000 Res. 2020;8. https://doi.org/10.12688/f1000research.18787.1.
Zarshenas N, Tapsell LC, Neale EP, Batterham M, Talbot ML. The relationship between bariatric surgery and diet quality: a systematic review. Obes Surg. 2020;30:1768–92.
Mechanick JI, Apovian C, Brethauer S, Timothy Garvey W, Joffe AM, Kim J, et al. Clinical practice guidelines for the perioperative nutrition, metabolic, and nonsurgical support of patients undergoing bariatric procedures – 2019 update: cosponsored by American association of clinical endocrinologists/american college of endocrinology, the obesity society, american society for metabolic and bariatric surgery, obesity medicine association, and american society of anesthesiologists. Obesity. 2020;28:O1–O58.
Tabesh MR, Maleklou F, Ejtehadi F, Alizadeh Z. Nutrition, physical activity, and prescription of supplements in pre- and post-bariatric surgery patients: a practical guideline. Obes Surg. 2019;29:3385–400.
Handzlik-Orlik G, Holecki M, Orlik B, Wyleżoł M, Duława J. Nutrition management of the post-bariatric surgery patient. Nutr Clin Pract. 2015;30:383–92.
Sarker A, Meek CL, Park A. Biochemical consequences of bariatric surgery for extreme clinical obesity. Ann Clin Biochem. 2016;53:21–31.
Boerlage TC, van de Laar AW, Westerlaken S, Gerdes VE, Brandjes DP. Gastrointestinal symptoms and food intolerance 2 years after laparoscopic Roux-en-Y gastric bypass for morbid obesity. Br J Surg. 2017;104:393–400.
Gobato RC, Cazzo E, Baltieri L, Modena DAO, Chaim EA. Food Intolerance 1 Year After Banded Roux-En-Y Gastric Bypass. Obes Surg. 2019;29:485–91.
Mosinski JD, Kirwan JP. Longer-term physiological and metabolic effects of gastric bypass surgery. Curr Diab Rep. 2016;16:50. https://doi.org/10.1007/s11892-016-0747-1
Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71.
Ouzzani M, Hammady H, Fedorowicz Z, Elmagarmid A. Rayyan — a web and mobile app for systematic reviews. Syst Rev. 2016;5:210. https://doi.org/10.1186/s13643-016-0384-4
Cohen, J. Statistical power analysis for the behavioral sciences [Internet]. 2nd ed. Routledge; 1988. https://doi.org/10.4324/9780203771587
Merlin T, Weston A, Tooher R, et al. NHMRC levels of evidence and grades for recommendations for developers of guidelines. In: Australian government national health and medical research council. 2009 https://www.nhmrc.gov.au/sites/default/files/images/NHMRC%20Levels%20and%20Grades%20 (2009).
Scottish Intercollegiate Guidelines Network. Checklists (SIGN. In: Scottish Intercollegiate Guidelines Network. https://www.sign.ac.uk/what-we-do/methodology/checklists/.
Alvarez V, Carrasco F, Cuevas A, Valenzuela B, Muñoz G, Ghiardo D, et al. Mechanisms of long-term weight recurrence in patients undergoing sleeve gastrectomy. Nutrition. 2016;32:303–8.
Amundsen T, Strømmen M, Martins C. Suboptimal weight loss and weight recurrence after gastric bypass surgery-postoperative status of energy intake, eating behavior, physical activity, and psychometrics. Obes Surg. 2017;27:1316–23.
Bobbioni-Harsch E, Huber O, Morel P, Chassot G, Lehmann T, Volery M, et al. Factors influencing energy intake and body weight loss after gastric bypass. Eur J Clin Nutr. 2002;56:551–6.
Cadena-Obando D, Ramírez-Rentería C, Ferreira-Hermosillo A, Albarrán-Sanchez A, Sosa-Eroza E, Molina-Ayala M, et al. Are there really any predictive factors for a successful weight loss after bariatric surgery? BMC Endocr Disord. 2020;20.
Chou JJ, Lee WJ, Almalki O, Chen JC, Tsai PL, Yang SH. Dietary intake and weight changes 5 years after laparoscopic sleeve gastrectomy. Obes Surg. 2017;27:3240–6.
Sherf Dagan S, Keidar A, Raziel A, Sakran N, Goitein D, Shibolet O, et al. Do bariatric patients follow dietary and lifestyle recommendations during the first postoperative year? Obes Surg. 2017;27:2258–71.
da Silva FBL, Gomes DL, de Carvalho KMB. Poor diet quality and postoperative time are independent risk factors for weight recurrence after Roux-en-Y gastric bypass. Nutrition. 2016;32:1250–3.
Dodsworth A, Warren-Forward H, Baines S. Feasibility of a protein-enriched diet after laparoscopic adjustable gastric banding: results from a pilot intervention. e-SPEN. Journal. 2012;7:e57–e63.
Faria SL, Faria OP, Lopes TC, Galvão MV, De Oliveira Kelly E, Ito MK. Relation between carbohydrate intake and weight loss after bariatric surgery. Obes Surg. 2009;19:708–16.
Faria S, De Oliveira Kelly E, Pereira Faria O, Kiyomi Ito M. Snack-eating patients experience lesser weight loss after roux-en-Y gastric bypass surgery. Obes Surg. 2009;19:1293–6.
Faria SL, De Oliveira Kelly E, Lins RD, Faria OP. Nutritional management of weight recurrence after bariatric surgery. Obes Surg. 2010;20:135–9.
Freire RH, Borges MC, Alvarez-Leite JI, Correia MITD. Food quality, physical activity, and nutritional follow-up as determinant of weight recurrence after Roux-en-Y gastric bypass. Nutrition. 2012;28:53–8.
Gallé F, Marte G, Cirella A, Di Dio M, Miele A, Ricchiuti R, et al. An exercise-based educational and motivational intervention after surgery can improve behaviors, physical fitness and quality of life in bariatric patients. PloS one. 2020;15:e0241336.
Iossa A, Coluzzi I, Giannetta IB, Silecchia G. Weight loss and eating pattern 7 years after sleeve gastrectomy: experience of a bariatric center of excellence. Obes Surg. 2020;30:3747–52.
Jassil FC, Manning S, Lewis N, Steinmo S, Kingett H, Lough F, et al. Feasibility and impact of a combined supervised exercise and nutritional-behavioral intervention following bariatric surgery: a pilot study. J Obes. 2015:693829. https://doi.org/10.1155/2015/693829.
Kalarchian MA, Marcus MD, Courcoulas AP, Lutz C, Cheng Y, Sweeny G. Structured dietary intervention to facilitate weight loss after bariatric surgery: a randomized, controlled pilot study. Obesity (Silver Spring, Md). 2016;24:1906–12.
Kanerva N, Larsson I, Peltonen M, Lindroos AK, Carlsson LM. Changes in total energy intake and macronutrient composition after bariatric surgery predict long-term weight outcome: findings from the Swedish obese subjects (SOS) study. Am J Clin Nutr. 2017;106:136–45.
Kruseman M, Leimgruber A, Zumbach F, Golay A. Dietary, weight, and psychological changes among patients with obesity, 8 years after gastric bypass. J. Am. Diet. Assoc. 2010;110:527–34.
Lim HS, Kim YJ, Lee J, Yoon SJ, Lee B. Establishment of adequate nutrient intake criteria to achieve target weight loss in patients undergoing bariatric surgery. Nutrients. 2020;12:1–12.
Lindroos AK, Lissner L, Sjöström L. Weight change in relation to intake of sugar and sweet foods before and after weight reducing gastric surgery. INT J OBES. 1996;20:634–43.
Masood A, Alsheddi L, Alfayadh L, Bukhari B, Elawad R, Alfadda AA. Dietary and lifestyle factors serve as predictors of successful weight loss maintenance postbariatric surgery. J Obes. 2019;7295978. https://doi.org/10.1155/2019/7295978.
Moizé V, Andreu A, Flores L, Torres F, Ibarzabal A, Delgado S, et al. Long-term dietary intake and nutritional deficiencies following sleeve gastrectomy or roux-en-y gastric bypass in a mediterranean population. J Acad Nutri Diet. 2013;113:400–10.
Novais PFS, Rasera I. Leite CVDS, Marin FA, De Oliveira MRM. Food intake in women two years or more after bariatric surgery meets adequate intake requirements. Nutr Res. 2012;32:335–41.
Ortega J, Ortega-Evangelio G, Cassinello N, Sebastia V. What are obese patients able to eat after Roux-en-Y gastric bypass? Obes Facts. 2012;5:339–48.
Palacio A, Luna C, Maíz C, Blanco E. Nutritional and behavioral factors related to weight gain after bariatric surgery. Revista Medica De Chile. 2021;149:30–6.
Papalazarou A, Yannakoulia M, Kavouras SA, Komesidou V, Dimitriadis G, Papakonstantinou A, et al. Lifestyle intervention favorably affects weight loss and maintenance following obesity surgery. Obesity. 2010;18:1348–53.
Pinto SL, Juvanhol LL, Bressan J. Increase in protein intake after 3 months of RYGB is an independent predictor for the remission of obesity in the first year of surgery. Obes Surg. 2019;29:3780–5.
Raftopoulos I, Bernstein B, O'Hara K, Ruby JA, Chhatrala R, Carty J. Protein intake compliance of morbidly obese patients undergoing bariatric surgery and its effect on weight loss and biochemical parameters. Surg Obes Relat Dis. 2011;7:733–42.
Reid RER, Oparina E, Plourde H, Andersen RE. Energy intake and food habits between weight maintainers and re-occurrenceers, five years after Roux-en-Y gastric bypass. Can J Diet Pract Res. 2016;77:195–8.
Ruiz-Lozano T, Vidal J, de Hollanda A, Scheer FAJL, Garaulet M, Izquierdo-Pulido M. Timing of food intake is associated with weight loss evolution in severe obese patients after bariatric surgery. Clin Nutr. 2016;35:1308–14.
Sarwer DB, Moore RH, Spitzer JC, Wadden TA, Raper SE, Williams NN. A pilot study investigating the efficacy of postoperative dietary counseling to improve outcomes after bariatric surgery. Surg Obes Relat Dis. 2012;8:561–8.
Schiavo L, Scalera G, Pilone V, De Sena G, Quagliariello V, Iannelli A, et al. A comparative study examining the impact of a protein-enriched vs normal protein postoperative diet on body composition and resting metabolic rate in obese patients after sleeve gastrectomy. Obes Surg. 2017;27:881–8.
Schiavo L, Favrè G, Pilone V, Rossetti G, De Sena G, Iannelli A, et al. Low-purine diet is more effective than normal-purine diet in reducing the risk of gouty attacks after sleeve gastrectomy in patients suffering of gout before surgery: a retrospective study. Obes Surg. 2018;28:1263–70.
Taus M, Fumelli D, Busni D, Borroni F, Sebastianelli S, Nicolai G, et al. A very low calorie ketogenic diet improves weight loss and quality of life in patients with adjustable gastric banding. Annali Italiani Di Chirurgia. 2017;88:S0003469X17026550.
Wardé-Kamar J, Rogers M, Flancbaum L, Laferrère B. Calorie intake and meal patterns up to 4 years after Roux-en-Y gastric bypass surgery. Obes Surg. 2004;14:1070–9.
Yanos BR, Saules KK, Schuh LM, Sogg S. Predictors of lowest weight and long-term weight recurrence among Roux-en-Y gastric bypass patients. Obes Surg. 2015;25:1364–70.
Cornejo-Pareja I, Molina-Vega M, Gómez-Pérez AM, Damas-Fuentes M, Tinahones FJ. Factors related to weight loss maintenance in the medium–long term after bariatric surgery: a review. J Clin Med. 2021;10.
Benson-Davies S, Davies ML, Kattelmann K. Energy balance following gastric bypass surgery: a pilot study of daily caloric intake and step count. Bariatr Surg Pract Patient Care. 2013;8:23–8.
Kostecka M, Bojanowska M. Problems in bariatric patient care-challenges for dieticians. Wideochir Inne Tech Maloinwazyjne. 2017;12:207–15.
Kushner RF, Sorensen KW. Prevention of weight recurrence following bariatric surgery. Curr Obes Rep. 2015;4:198–206.
Forbush S, Nof L, Echternach J, Hill C, Rainey J. Influence of activity levels and energy intake on percent excess weight loss after Roux-en-Y gastric bypass. Obes Surg. 2011;21:1731–8.
Novaes Ravelli M, Schoeller DA, Crisp AH, Shriver T, Ferriolli E, Ducatti C, et al. Influence of energy balance on the rate of weight loss throughout one year of roux-en-y gastric bypass: a doubly labeled water study. Obes Surg. 2019;29:3299–308.
Pilkington TR, Gazet JC, Ang L, Kalucy RS, Crisp AH, Day S. Explanations for weight loss after ileojejunal bypass in gross obesity. Br Med J. 1976;1:1504–5.
Coen PM, Carnero EA, Goodpaster BH. Exercise and bariatric surgery: an effective therapeutic strategy. Exerc Sport Sci Rev. 2018;46:262–70.
Ito MK, Gonçalves VSS, Faria SLCM, Moizé V, Porporatti AL, Guerra ENS. et al. Effect of protein intake on the protein status and lean mass of post-bariatric surgery patients: a systematic review. Obes Surg. 2017;27:502–12. https://doi.org/10.1007/s11695-016-2453-0.
Romeijn MM, Holthuijsen DDB, Kolen AM, Janssen L, Schep G, van Dielen FMH, et al. The effect of additional protein on lean body mass preservation in post-bariatric surgery patients: a systematic review. Nutr J. 2021;20:27 https://doi.org/10.1186/s12937-021-00688-3.
Gil S, Goessler K, Dantas WS, Murai IH, Merege-Filho CAA, Pereira RMR, et al. Constraints of weight loss as a marker of bariatric surgery success: an exploratory study. Front Physiol. 2021;12:640191 https://doi.org/10.3389/fphys.2021.640191.
McGrice MA, Porter JA. What are gastric banding patients eating one year post-surgery? Obes Surg. 2012;22:1855–8.
Harbury C, Collins CE, Callister R. Diet quality is lower among adults with a BMI ≥40 kgm−2 or a history of weight loss surgery. Obes Res Clin Pract. 2019;13:197–204.
Soares FL, Bissoni de Sousa L, Corradi-Perini C, Ramos da Cruz MR, Nunes MG, Branco-Filho AJ. Food quality in the late postoperative period of bariatric surgery: an evaluation using the bariatric food pyramid. Obes Surg. 2014;24:1481–6.
Freeman RA, Overs SE, Zarshenas N, Walton KL, Jorgensen JO. Food tolerance and diet quality following adjustable gastric banding, sleeve gastrectomy and Roux-en-Y gastric bypass. Obes Res Clin Pract. 2014;8:e183–e91.
Hubert PA, Papasavas P, Stone A, Swede H, Huedo-Medina TB, Tishler D, et al. Associations between weight loss, food likes, dietary behaviors, and chemosensory function in bariatric surgery: a case-control analysis in women. Nutrients. 2019;11:804.
Tan MS, Cheung HC, McAuley E, Ross LJ, MacLaughlin HL. Quality and validity of diet quality indices for use in Australian contexts: a systematic review. Br J Nutr. 2022;128:2021–45.
McAuley EA, MacLaughlin HL, Hannan-Jones MT, King N, Ross LJ. Effectiveness of diet quality indices in measuring a change in diet quality over time: a systematic review and meta-analysis of randomized controlled trials. Nutr Rev. 2023;81:361–83.
Gils Contreras A, Bonada Sanjaume A, Becerra-Tomás N, Salas-Salvadó J. Adherence to mediterranean diet or physical activity after bariatric surgery and its effects on weight loss, quality of life, and food tolerance. Obes Surg. 2020;30:687–96.
Rebelos E, Moriconi D, Honka MJ, Anselmino M, Nannipieri M. Decreased weight loss following bariatric surgery in patients with type 2 diabetes. Obes Surg. 2023;33:179–87.
Majid SF, Davis MJ, Ajmal S, Podkameni D, Jain-Spangler K, Guerron AD, et al. Current state of the definition and terminology related to weight recurrence after metabolic surgery: review by the POWER Task Force of the ASMBS. Surg Obes Relat Dis. 2022;18:957–63.
Rennie KL, Coward A, Jebb SA. Estimating under-reporting of energy intake in dietary surveys using an individualised method. Br J Nutr. 2007;97:1169–76.
Acknowledgements
The authors wish to acknowledge the Queensland University of Technology and the Royal Brisbane and Women’s Hospital for their support of this research.
Funding
This research was part of HC’s Doctor of Philosophy candidacy and has received no specific grant from any funding agency, commercial or not-for-profit sectors. Open Access funding enabled and organized by CAUL and its Member Institutions.
Author information
Authors and Affiliations
Contributions
HC, LR, ES, and JM contributed to the conceptualization of this research question and eligibility criteria. Literature search and abstract/title screening was completed by HC, where full text screening was completed by HC, LR, ES, JM, and HM. Quality and risk of bias assessments were led by HC and cross-checked by LR. Data synthesis and the drafts of the review were led and written by HC and reviewed by all authors. All authors have agreed with the final manuscript being submitted and declare that the manuscript has not been published elsewhere.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Cheung, H.C., Strodl, E., Musial, J. et al. Associations between diet composition, dietary pattern, and weight outcomes after bariatric surgery: a systematic review. Int J Obes 47, 764–790 (2023). https://doi.org/10.1038/s41366-023-01333-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41366-023-01333-1