Abstract
Objective
To estimate the association between childhood obesity and the risk of SARS-CoV-2 infection in a cohort followed from 4 to 12 years of age.
Methods
The data were obtained from two independent sources: the Longitudinal Childhood Obesity Study (ELOIN) and the epidemiological surveillance system data from the Community of Madrid (Spain), which served to identify the population within the cohort with confirmed SARS-CoV-2 infection. The SARS-CoV-2 registry was cross-checked with the cohort population at 11–12 years of age. A total of 2018 eligible participants were identified in the cohort, who underwent physical examinations at 4, 6, and 9 years of age during which weight, height, and waist circumference were recorded. General obesity (GO) was determined according to the WHO-2007 criteria whereas abdominal obesity (AO) was defined based on the International Diabetes Federation (IDF) criteria. The relative risks (RRs) of infection were estimated using a Poisson regression model and adjusted by sociodemographic variables, physical activity, and perceived health reported by the parents.
Results
The accumulated incidence of SARS-CoV-2 infection was 8.6% (95% CI: 7.3–9.8). The estimated RR of SARS-CoV-2 infection was 2.53 (95% CI: 1.56–4.10) and 2.56 (95% CI: 1.55–4.21) for children 4–9 years old with stable GO and AO, respectively, compared with those who did not present GO.
Conclusions
Childhood obesity is an independent risk factor for SARS-CoV-2 infection. This study provides new evidence that indicates that obesity increases the vulnerability of the paediatric population to infectious diseases.
Similar content being viewed by others
Introduction
Infection with SARS-CoV-2 causes COVID-19, a disease that has spread rapidly worldwide to become a pandemic that has caused an unprecedented public health crisis. In May 2021, there were more than 155 million cases and over three million deaths worldwide [1], with significant variations in incidence depending on geographical areas. With more than 3 million confirmed cases in May 2021, Spain has been one of the most affected European countries since the beginning of the health emergency, with the Community of Madrid being one of the regions with the highest incidence and mortality in the Spanish territory [2].
Although a significant proportion of infected individuals are asymptomatic or show mild symptoms, the moderate and severe forms of the disease have prevalence rates that can exceed 50% [3, 4]. SARS-CoV-2 infection in children and adolescents has been characterised by less severe cases [1, 5] than in the adult population, occurring more frequently in an asymptomatic or mild form [6].
Overweight and obesity are risk factors for greater disease severity, characterised by the need for hospitalisation, admission to intensive care units, and requiring mechanical ventilation [7,8,9], especially for patients with severe obesity [10,11,12]. Although the evidence on the association of COVID-19 severity and obesity is stronger in the adult population [13, 14], it has also been described in the paediatric population [15]. Obesity and impaired metabolic health may correlate with an increased risk of severe COVID-19 [16]. Different immunological mechanisms have been proposed to explain the increased risk of complications in this population group [13, 17, 18], although many issues remain unresolved [19].
The association of excess weight (overweight and obesity) with an increased risk of infection is a controversial aspect with limited information. The greater frequency of both complications and presence of symptoms in people with this condition can increase the probability of detection and result in a greater number of tests, as has been reported in previous studies conducted in the adult population [14]. A study in middle-aged adults based on data from the United Kingdom (Biobank) documented the existence of a dose-response association between body mass index (BMI) and waist circumference with testing positive for SARS-CoV-2 [20]. Another study cross-referenced a paediatric population in Mexico with COVID-19 surveillance records and found obesity to be one of the predictors associated with a positive laboratory test [21].
The objective of this study was to estimate the risk of SARS-CoV-2 infection associated with childhood obesity, both general and abdominal, from a cohort of children followed from 4 to 12 years of age.
Methods
Design and study population
A cohort study was conducted by extracting data from two different, independent sources: the Longitudinal Childhood Obesity Study (ELOIN study) and the SARS-CoV-2 epidemiological surveillance system for the Community of Madrid (Spain), a region with a population of 6,779,888 inhabitants in 2020. Both databases were cross-checked by means of the personal public health identification number. The ELOIN study is a population-based cohort study in the Community of Madrid with a follow-up period beginning in 2012 and whose methodology has been previously described and published [22]. The cohort included 2018 participants who underwent physical examinations at the ages of 4 years (2012–2013), 6 years (2014–2015), and 9 years (2017–2018). Measurements were recorded at the follow-up visits through standardised physical examinations performed by paediatricians at primary care centres and additional information was obtained through a structured questionnaire answered by the parents via a computer-assisted telephone interview.
Anthropometric measurements
Weight was measured with a digital scale (SECA® model 220, precision of 0.1 kg), and height was measured with a telescopic stadiometer (SECA® model 220, precision of 1 mm). For measuring the waist circumference, an approved flexible measuring tape with a buckle was used, placed horizontally right above the iliac crest and without tissue compression. Two measurements per participant were taken and the average was calculated for the analyses.
BMI was calculated in kg/m2 and adjusted by age (months) and sex according to standardised tables of the WHO-2007 [23]. Based on BMI z-scores in terms of standard deviation (SD), underweight was defined zBMI ≤2 DE, normal weight was defined as zBMI ≤ 1 SD, overweight was defined as 1 SD <zBMI ≤ 2 SD, and general obesity (GO) was defined as zBMI > 2 SD [11]. The waist circumference was standardised by age (months) and sex according to reference tables generated from the consensus definition of metabolic syndrome by the International Diabetes Federation, and the cut-off point for abdominal obesity (AO) was set at ≥90th percentile [24].
Based on the weight status evolution at 4, 6, and 9 years of age, participants were classified as (1) stable without obesity (i.e., without obesity at all three measurements); (2) transient obesity (i.e., obesity at some point of the cohort follow-up period); and (3) stable with obesity (i.e., obesity at all three measurements). This classification was used to evaluate changes in both GO and AO.
Definition of SARS-CoV-2 infection
The SARS-CoV-2 infection status was determined for all cohort participants. Subjects were classified as infected when they had received a diagnosis of active infection with a positive viral RNA detection test by RT-PCR or an equivalent molecular technique or a rapid antigen detection test (Ag-RDT) during the pandemic and were reported in the epidemiological surveillance system of the Community of Madrid before May 9th of 2021. The epidemiological surveillance system is a database of all confirmed cases of SARS-CoV-2 infection created within the Strategy of early detection vigilance and control of COVID-19 in the Community of Madrid (Estrategia de detección precoz, vigilancia y control de COVID-19 de la Comunidad de Madrid) [25], which includes epidemiological, clinical, microbiological, and evolutionary data.
Covariates
The following variables, which were obtained from the 9-year ELOIN study, were included: sex; age (months); family affluence estimated with the Family Affluence Scale (FAS) and classified as low (0–3 points), medium (4–5 points), and high (6–9 points) [26, 27]; household size; physical activity estimated via the Spanish-validated version of the Physical Activity Questionnaire—Children [28], with scores ranging between 1 (low physical activity) and 5 (high physical activity) and classified into quartiles; perceived health status (very good/good, fair/poor/very poor); and parents’ perception of neighbourhood environment (very good/good, fair/bad/very bad).
Statistical analysis
A descriptive analysis was performed. Quantitative variables were represented by their mean (SD) values and qualitative variables by percentages with their respective confidence intervals. The statistical associations between qualitative variables were evaluated with the chi-squared test (χ2) and the comparisons of means with the Student’s t test. Subsequently, Poisson regression models were developed to estimate robust variance of the likelihood (relative risk, RR) of SARS-CoV-2 infection in relation to the evolution of GO and AO. Crude estimations and adjusted models were generated for sex, age, family purchasing power, household size, physical activity, perceived health status, and parents’ perception of neighbourhood environment, introducing all variables simultaneously. Additionally, the associations of both GO and AO with the risk of SARS-CoV-2 infection were explored disaggregating the data by sex.
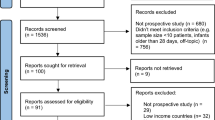
Of the initial 2018 eligible participants, 33 were classified as underweight and were therefore excluded. An additional 41 observations were excluded from the AO analysis due to the lack of measurements of waist circumference. Only participants with anthropometric measurement data (BMI) collected at the three follow-up periods were included in the analysis.
A sensitivity analysis was performed by assessing the risk of infection using the same methodology for 665 participants with available BMI measurements and 656 subjects with available waist circumference records through the ages of 4 to12 years, and who completed the questionnaire at the age of 12 years when this study was conducted.
All analyses were performed with software Stata v.16 (StataCorp, College Station, USA).
Results
A total of 1985 and 1944 participants with available BMI and waist circumference measurements, respectively, were included in the analyses. The accumulated incidence of SARS-CoV-2 infection was 8.6% (95% CI: 7.3‒9.8) and the mean age at the time of infection was 11.9 years (SD 0.23). Table 1S (Supplementary Material) shows the distribution of general and abdominal obesity at 4, 6 and 9 years of age according to SARS-CoV-2 infection status of the cohort. The infection was classified as mild in 49.4% of participants (n = 84), one participant required hospitalisation, and none required admission to an intensive care unit. The remaining participants presented asymptomatic infection. The most frequently reported symptoms were fever, headache, myalgia, and cough. A total of 3.5% (n = 6) of the infected participants reported a history of chronic disease, and 6.3% (n = 11) of the infections occurred during pandemic outbreaks
The characteristics of the population sample in terms of SARS-CoV-2 infection status are presented in Table 1. No statistically significant differences in the assessed variables were observed, except for the frequency of obesity, both GO and AO, with a two- to three-fold higher prevalence of stable obesity in infected versus uninfected children. Tables 2S and 3S of the Supplementary data show the relationship between of the study population and transitions from GO and AO in the cohort.
Table 2 shows the association between the risk of SARS-CoV-2 infection at 12 years of age and general and abdominal obesity from 4 to 9 years of age adjusted for the main potentially confounding variables. Compared to children without obesity during the follow-up period, the RR of SARS-CoV-2 infection was 2.5 (95% CI: 1.5‒4.1) and 2.5 (95% CI: 1.5‒4.2) for the cohort participants with stable GO or with AO, respectively. Although the risk increased in children with GO and transient AO, statistical significance was not reached.
A sensitivity analysis was performed with the partial data of participants who underwent a physical examination and completed the questionnaire at 12 years (Table 4S of Supplementary Material). The risk of infection in children with persistent GO was 2.8 (95% CI: 0.8‒9.3) and reached up to 3.7 (95% CI: 1.2–10.9) in children with persistent AO.
No statistically significant relationships were observed between sex and the association of GO and AO with the risk of SARS-CoV-2 infection.
Discussion
This study describes the association of obesity with the risk of SARS-CoV-2 infection using data from a cohort of children followed from 4 to 12 years of age. The results indicate an increased risk of SARS-CoV-2 infection in participants with persistent GO and/or AO between 4 and 9 years of age compared with children not presenting obesity throughout the follow-up period.
Obesity has been related to severe COVID-19 and presents a wide spectrum of clinical manifestations in the child population [15]. The largest proportion of participants in this study presented asymptomatic infection or mild symptoms and the proportion of children with symptoms among those with stable obesity was not higher than among those classified as stable without obesity. On the other hand, an association between increased risk of infection and obesity was observed. This finding suggests a relationship between SARS-CoV-2 infection and obesity in the paediatric population is independent of patient perceived manifestation of the infection.
In the analysis of the evolution of GO and AO until 9 years of age, the risk of infection was found to be almost three times higher in children with persistent GO and AO, compared to those without GO and AO, suggesting a greater vulnerability to infection in children with persistent obesity. The evidence in the literature describing the association between obesity and the risk of SARS-CoV-2 infection is very limited. A study conducted in the United Kingdom in middle-aged adults described a dose-response relationship between BMI and waist circumference with a positive test for SARS-CoV-2, with an estimated odds ratio of 1.5 for risk of infection in people with obesity [20]. In another study conducted in Mexico that was based on cross-referenced records of COVID-19 from national surveillance data and a cohort of children and adolescents <15 years of age, obesity was one of the predictors associated with a positive laboratory test, with an estimated odds ratio of 2 [21].
The outcomes of the present study also showed that long-term obesity entails a higher risk of infection, since participants with persistent obesity presented almost two-fold the risk of infection compared to those who were classified with obesity only in the last follow-up period (9 years of age). This could be explained by the altered immunological mechanisms found in individuals with persistent obesity, which could result in increased vulnerability to infection. Moreover, obesity is commonly characterised as a state of low-grade inflammation [29] that alters innate and adaptive immune responses [30] against certain pathogens and potentially impacts on the response to vaccination [17].
Limitations and strengths of the study
The following limitations should be taken into account when interpreting the results of this study. First, this work drew data from the SARS-CoV-2 epidemiological surveillance system of the Community of Madrid, which includes children who were tested for infection because of showing COVID-19 symptoms or being close contacts to a confirmed case. Therefore, many asymptomatic cases were likely undetected and not included in this database. However, the resulting bias would be non-differential since the proportion of children in this registry infected with SARS-CoV-2 with symptoms did not vary depending on their obesity status; the distribution of symptoms in children with SARS-CoV-2 infection according to GO and AO transitions is shown in supplementary material (Tables 5S and 6S). Second, no information was obtained on weight status between birth and 4 years of age, which prevents the completion of obesity trajectories since birth. Third, the anthropometric measurements were only recorded until 9 years of age. However, the results of the sensitivity analysis in the subset of children with available measurements of BMI and waist circumference at the age of 12 years were consistent with those observed until 9 years of age. Fourth, besides the FAS, there was information on parents’ level of education and immigrant status. However, these variables had lower response rate, so we kept FAS to estimate the economic level of the families. Nevertheless, the estimations after adjusting for the educational level of the children’s mother and immigrant status were very similar, but the number of participants was much lower (Tables 7S and 8S of Supplementary Material). Five, for some of the analyses, the number of participants was small, and replication of findings in future research would therefore be beneficial. Finally, a moderate selection bias was observed in the ELOIN cohort that affects its population representativeness: the response rate at the baseline evaluation was lower for children with low education levels and foreign parents [22], characteristics that have also been associated with greater vulnerability to SARS-CoV-2 infection [31]. However, children who attended the three main follow-up assessments in this study fit the general sociodemographic characteristics of the baseline cohort despite the losses to follow-up (Table 9S of Supplementary Material).
Among the strengths of the study is the longitudinal design and the availability of information on the main confounding variables. In addition, the sample was representative of the general population, even with the above-mentioned selection bias. Furthermore, the information obtained from the surveillance records provided clinical and epidemiological information that allowed to characterise the infection in the population under study.
In conclusion, obesity is an independent risk factor for SARS-CoV-2 infection in the paediatric population. Therefore, the individuals in this population with this health condition should be considered a vulnerable group, and necessary measures should be adopted for the prevention, intervention, and early management of infection to avoid the development of more severe forms of COVID-19 or the appearance of complications.
Summary
What is known on this subject
-
Obesity, and to a greater extent severe obesity, are associated with more severe clinical evolution of SARS-CoV-2 infection and an increased risk of requiring hospitalisation or admission to the ICU.
What this study adds
-
The study, which is based on a population-based cohort of children, shows that general and abdominal persistent obesity are associated with an increased risk of SARS-CoV-2 infection.
References
World Health Organization. Coronavirus (COVID-19). 2021. https://covid19.who.int.
Ministerio de Sanidad. Situación actual Coronavirus (COVID-19). 2021. https://www.mscbs.gob.es/profesionales/saludPublica/ccayes/alertasActual/nCov/situacionActual.htm.
Suleyman G, Fadel RA, Malette KM, Hammond C, Abdulla H, Entz A, et al. Clinical characteristics and morbidity associated with Coronavirus Disease 2019 in a series of patients in metropolitan detroit. JAMA Netw Open. 2020;3:e2012270.
Paranjpe I, Russak AJ, De Freitas JK, Lala A, Miotto R, Vaid A, et al. Retrospective cohort study of clinical characteristics of 2199 hospitalised patients with COVID-19 in New York City. BMJ Open. 2020;10:e040736.
Nathan N, Prevost B, Sileo C, Richard N, Berdah L, Thouvenin G, et al. The wide spectrum of COVID-19 clinical presentation in children. J Clin Med. 2020;9:2950.
Siebach MK, Piedimonte G, Ley SH. COVID-19 in childhood: transmission, clinical presentation, complications and risk factors. Pediatr Pulmonol. 2021;56:1342–56.
Popkin BM, Du S, Green WD, Beck MA, Algaith T, Herbst CH, et al. Individuals with obesity and COVID-19: a global perspective on the epidemiology and biological relationships. Obes Rev. 2020;21:e13128.
Zhang X, Lewis AM, Moley JR, Brestoff JR. A systematic review and meta-analysis of obesity and COVID-19 outcomes. Sci Rep. 2021;11:7193.
Helvaci N, Eyupoglu ND, Karabulut E, Yildiz BO. Prevalence of obesity and its impact on outcome in patients with COVID-19: a systematic review and meta-analysis. Front Endocrinol. 2021;12:598249.
Ko JY, Danielson ML, Town M, Derado G, Greenlund KJ, Daily Kirley P, et al. Risk factors for COVID-19-associated hospitalization: COVID-19-associated hospitalization surveillance network and behavioral risk factor surveillance system. Clin Infect Dis. 2021;72:e695–703.
Kompaniyets L, Goodman AB, Belay B, Freedman DS, Sucosky MS, Lange SJ, et al. Body mass index and risk for COVID-19-related hospitalization, intensive care unit admission, invasive mechanical ventilation, and death—United States, March-December 2020. MMWR Morb Mortal Wkly Rep. 2021;70:355–61.
Centers for Disease Control and Prevention. COVID-19 and Your Health. 2020. https://www.cdc.gov/coronavirus/2019-ncov/need-extra-precautions/people-with-medical-conditions.html.
Okauchi Y, Matsuno K, Nishida T, Sawada K, Kawasaki A, Ito N, et al. Obesity, glucose intolerance, advanced age, and lymphocytopenia are independent risk factors for oxygen requirement in Japanese patients with Coronavirus disease 2019 (COVID-19). Endocr J. 2021. https://doi.org/10.1507/endocrj.EJ20-0784.
Chadeau-Hyam M, Bodinier B, Elliott J, Whitaker MD, Tzoulaki I, Vermeulen R, et al. Risk factors for positive and negative COVID-19 tests: a cautious and in-depth analysis of UK biobank data. Int J Epidemiol. 2020;49:1454–67.
Zachariah P, Johnson CL, Halabi KC, Ahn D, Sen AI, Fischer A, et al. Epidemiology, clinical features, and disease severity in patients with Coronavirus Disease 2019 (COVID-19) in a Children’s Hospital in New York City, New York. JAMA Pediatrics. 2020;174:e202430.
Stefan N, Birkenfeld AL, Schulze MB. Global pandemics interconnected—obesity, impaired metabolic health and COVID-19. Nature Reviews Endocrinology. 2021;17:135–49.
Albashir AAD. The potential impacts of obesity on COVID-19. Clin Med. 2020;20:e109–13.
Fang X, Henao-Mejia J, Henrickson SE. Obesity and immune status in children. Curr Opin Pediatr. 2020;32:805–15.
Flint SW, Tahrani AA. COVID-19 and obesity-lack of clarity, guidance, and implications for care. Lancet Diabetes Endocrinol. 2020;8:474–5.
Yates T, Razieh C, Zaccardi F, Davies MJ, Khunti K. Obesity and risk of COVID-19: analysis of UK biobank. Prim Care Diabetes. 2020;14:566–7. https://doi.org/10.1016/j.pcd.2020.05.011.
Murillo-Zamora E, Aguilar-Sollano F, Delgado-Enciso I, Hernandez-Suarez CM. Predictors of laboratory-positive COVID-19 in children and teenagers. Public Health. 2020;189:153–7.
Ortiz-Marron H, Cuadrado-Gamarra JI, Esteban-Vasallo M, Cortes-Rico O, Sanchez-Diaz J, Galan-Labaca I. The Longitudinal Childhood Obesity Study (ELOIN): design, participation and characteristics of the baseline sample. Rev Esp Cardiol. 2016;69:521–3.
de Onis M, Onyango AW. WHO child growth standards. Lancet. 2008;371:204.
Zimmet P, Alberti KGM, Kaufman F, Tajima N, Silink M, Arslanian S, et al. The metabolic syndrome in children and adolescents—an IDF consensus report. Pediatr Diabetes. 2007;8:299–306.
Consejería de Sanidad de la Comunidad de Madrid. Estrategia de Detección Precoz, Vigilancia y Control De Covid-19 de la Comunidad De Madrid. 2021. https://www.comunidad.madrid/sites/default/files/doc/sanidad/epid/estrategia_vigilancia_y_control_cm_22122021_f.pdf.
Torsheim T, Cavallo F, Levin KA, Schnohr C, Mazur J, Niclasen B, et al. Psychometric Validation of the revised family affluence scale: a latent variable approach. Child Indic Res. 2016;9:771–84.
ter Bogt TFM, de Looze M, Molcho M, Godeau E, Hublet A, Kokkevi A, et al. Do societal wealth, family affluence and gender account for trends in adolescent cannabis use? A 30 country cross-national study. Addiction. 2014;109:273–83.
Manchola-González J, Bagur-Calafat C, Girabent-Farrés M. Fiabilidad de la versión española del cuestionario de actividad física PAQ-C/Reliability Spanish Version of Questionnaire of Physical Activity PAQ-C. Rev Int Med Cienc Ac Fís Deporte. 2017;17:139–52.
Michalakis K, Ilias I. SARS-CoV-2 infection and obesity: common inflammatory and metabolic aspects. Diabetes Metab Syndr. 2020;14:469–71.
Guest PC. Clinical, biological and molecular aspects of COVID-19. Springer Nature. 2021;1321:356.
de Lusignan S, Dorward J, Correa A, Jones N, Akinyemi O, Amirthalingam G, et al. Risk factors for SARS-CoV-2 among patients in the Oxford Royal College of General Practitioners Research and Surveillance Centre primary care network: a cross-sectional study. Lancet Infect Dis. 2020;20:1034–42.
Acknowledgements
We would like to thank all the following participants: the families; the paediatricians belonging to the Madrid Regional Sentinel Primary care Physician Network. This study was funded by the Foundation for Biosanitary Research and Innovation in Primary Care (FIIBAP) and the Regional Ministry of Health of the Community of Madrid through non-refundable grants from the credits awarded to the Community of Madrid by the Spanish Government Fund COVID-19, included in Order HAC/667/2020.
Author information
Authors and Affiliations
Contributions
MAO-P, SdM-G, AO-T, and IG conceptualised and designed the study, drafted the initial manuscript, and reviewed the manuscript. HO-M and GC designed the data collection instruments, collected data, carried out the initial analyses. LFG–F, MO-G, and CQ-F critically reviewed the manuscript. All authors approved the final manuscript as submitted and agree to be accountable for all aspects of the work.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethical approval
The study protocol was approved by the Ethics Committee of Hospital Ramón y Cajal (Madrid, Spain). The parents of the participants provided written informed consent for their inclusion in the study.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Ortiz-Pinto, M.A., de Miguel-García, S., Ortiz-Marrón, H. et al. Childhood obesity and risk of SARS-CoV-2 infection. Int J Obes 46, 1155–1159 (2022). https://doi.org/10.1038/s41366-022-01094-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41366-022-01094-3