Abstract
The current pandemic due to widespread SARS-CoV-19 infection has again highlighted the role of obesity, whose global prevalence increased up to 13%, as a risk factor for both susceptibility to infections and the occurrence of a more severe disease course. To date, this association has not been sufficiently explored. Obesity-related susceptibility to infectious diseases is mostly thought to be due to an impairment of both innate and adaptive immune responses and vitamin D deficiency. Several cofactors can indirectly favour the onset and/or worsening of infectious diseases, such as impairment of respiratory mechanics, skin and subcutaneous tissue homoeostasis, obesity-related comorbidities and inappropriate antimicrobial therapy. Subjects with obesity have a higher incidence of cutaneous infections, probably due to changes in skin barrier functions and wound healing. Excess weight is also associated with an increased risk of urinary tract infection and its recurrence, as well as with a higher prevalence of both lower and higher respiratory tract infections. Moreover, patients with obesity appear to have an increased risk of surgical site infections when undergoing general, orthopaedic, gynaecological, and bariatric surgery. Data concerning the different infectious diseases related to obesity are rather limited since anthropometric parameters are usually poorly recorded. Furthermore, specific therapeutic protocols in subjects with obesity are lacking, especially regarding antibiotic therapy and further supplements. This review summarizes etiopathogenetic and epidemiological evidence and highlights areas of uncertainty in the field of infectious diseases and obesity, which require further research. It is important to raise public awareness of this additional risk related to obesity and to raise awareness among the scientific community to develop specific clinical protocols for subjects with obesity.
Similar content being viewed by others
Introduction
According to a recent estimate from the World Health Organization (WHO), at least 2.8 million of people die per year due to complications related to overweight or obesity (https://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight). Moreover, an estimated 35.8 million (2.3%) of global Disability-Adjusted Life Years (DALYs) are caused by overweight or obesity (https://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight). Worldwide, obesity has nearly tripled since 1975: currently, more than 1.9 billion adults are overweight, and more than 650 million of them are obese (39% of adults are overweight and 13% obese, according to the WHO) (https://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight), although obesity is defined as a real pandemic (https://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight).
In the era of a serious health crisis due to the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) pandemic, emerging data have demonstrated that obesity is a risk factor for infections and worsens the course and prognosis of diseases, leading to higher mortality rates [1]. Considering the above, an understanding of both mechanisms underlying the association between obesity and infectious diseases and the identification of more effective treatment strategies is urgently needed. By examining data on the incidence and prognosis of the most common infections in subjects with obesity, in regard to the flu, several meta-analyses and epidemiological studies disclosed an association between obesity and more severe prognosis, increased risk of admission to the intensive care unit (ICU) and death in subjects with H1N1 (Table 1); however, a higher incidence of infection in subjects with obesity has not been demonstrated [2,3,4,5,6]. Several other recent studies have shown an association between obesity and an increased risk of urinary tract infection (UTI), especially posttraumatic, ICU-acquired, pregnancy-related and postpartum [7,8,9,10]. Different cohort studies reported a close relation between obesity and the risk of nosocomial infections [11, 12], skin and surgical site infections (SSIs) [13,14,15,16], longer hospitalization and higher incidence rates of sepsis [17, 18]. Obesity is a risk factor for infectious pancreatitis [19], including its severe forms, and it has been shown to predispose patients to local complications such as pancreatic pseudocysts, abscesses and necrosis [20]. Several studies have suggested that obesity is associated with a wide range of skin diseases, such as cellulitis and erysipelas [21, 22], and with recurrent soft-tissue infections or prolonged hospitalization. However, regarding the prognosis, there is limited evidence indicating that the outcomes of cellulitis are worse in subjects with morbid obesity [23]. Obesity also carries an increased risk of hepatic steatosis in patients with chronic hepatitis C virus (HCV) infection, and it has been shown to have an adverse effect on the progression of chronic HCV liver disease, with diminished response to antiviral therapy [24]. It is very interesting to note that body mass index (BMI) seems to influence susceptibility to infections following a U-shaped trend [25]. In a prospective Danish cohort, 75,001 middle-aged women were monitored over a median time period of 11.9 years to evaluate the association between BMI and hospitalization and/or treatment for acute infections. The results indicated that the risk of overall and respiratory and skin infections was U-shaped, showing that both underweight and obesity can favour community-acquired infections [25]. These data were supported by a meta-analysis of 25 studies investigating 2.5 million adults and children older than 12 years living in industrialized countries that also demonstrated a U-shaped relationship between BMI and the risk of flu-related pneumonia [26]. Additionally, in elderly patients already more prone to infections and factors weakening the immune system, an overall increased risk of infection in subjects aged >75 years, both underweight (BMI < 20 kg/m2) and overweight (BMI > 28 kg/m2), was found. This finding suggests that BMI is a predictor for infectious disease risk regardless of age [27]. Therefore, the purpose of this review is to identify the possible etiopathogenetic mechanisms underlying the association between obesity and infectious diseases and then analyse the role of obesity in the context of infections in different body regions.
Search strategies
Articles were individually retrieved by each author up to February 2021 by searching PubMed (MEDLINE) using the following search terms: ‘obesity’, ‘immune system’, ‘infectious diseases’, ‘COVID-19’, ‘respiratory tract infections’, ‘urinary tract infections’, ‘skin infections’, ‘surgical site infections’, ‘vitamin D deficiency’, ‘type 2 diabetes mellitus’, and ‘H1N1 flu’. The reference lists of relevant articles and reviews were also searched manually.
Mechanisms involved in the increased susceptibility to infections in obesity
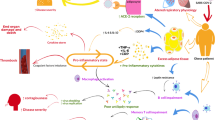
The mechanisms underlying the increased susceptibility to different types of infections in patients with obesity are not well established; however, some potential risk factors may be directly involved, including obesity-related alterations of the immune system and vitamin D deficiency. There are also some cofactors often associated with obesity that, even without a clear cause−effect relationship, can indirectly favour the onset or worsening of infectious diseases. These cofactors include changes in respiratory physiology, alterations in skin and soft tissue, comorbidities such as type 2 diabetes mellitus (T2DM) and cardiovascular disease (CVD), drug therapy, and, above all, the underdosing of antibiotics (Fig. 1).
Various mechanisms (red) have been postulated to contribute to increased infectious susceptibility in subjects with obesity, including alterations in respiratory mechanics and vitamin D deficiency and some cofactors (green) as alterations in homoeostasis of the skin and subcutaneous tissue, impairment of both innate and adaptive immune responses, the presence of obesity-related comorbidities and limitations relating to antimicrobial therapy.
Immune system
It has been shown that obesity has substantial effects on the immune system, probably because of the structural and functional similarity between immune system cells and adipocytes, both capable of producing mediators such as cytokines, chemokines and adipokines [28, 29]. Excess adipose tissue has been assimilated to a state of chronic inflammation, also known as ‘low-grade inflammation’, with consequences both locally on the adipose tissue itself—causing cell necrosis and dysfunction—and at the systemic level—altering the immune response [30]. In obesity, visceral adipose tissue produces an excess of cytokines such as tumour necrosis factor α (TNFα) and interleukin (IL) 6 and 1β that could weaken the response of immune cells during an infectious stimulus [30, 31]. In addition, in a state of excess weight, a condition of hyperleptinemia is observed that can contribute to an immune imbalance. Indeed, most innate immune cells express the leptin receptor [32]. The monocyte line, for example, produces greater amounts of IL-6, IL-12 and TNFα in response to leptin, while polymorphonuclear neutrophils pretreated with leptin produce twice as many reactive oxygen species (ROS) compared to those not treated [33, 34]. The adaptive immune system is also affected by leptin stimulation because leptin regulates both lymphopoiesis and myelopoiesis, as shown by a study in which ob/ob mice had fewer bone marrow cells than wild-type mice in response to leptin injection [35]. In particular, pre-B and immature B cells decreased by 21% and 12%, respectively, while there was a minor impact on myeloid cells [35]. Leptin also plays a role by stimulating activators of CD4 and CD8 T cells [36]. More recently, an effect on Treg proliferation and function was observed by De Rosa et al. who showed that Treg cells can increase their proliferation in the absence of leptin; thus, leptin resistance leads to an immunosuppressive phenotype. During excess weight, a leptin-resistant condition can develop, so its regulatory role in the immune system decreases, thus exposing the patient with obesity to a deficient immune response to infections. Obesity is also characterized by hyperinsulinaemia and insulin resistance. Monocytes express the insulin receptor; nevertheless, it is not clear how it affects their activity [37]. T cells do not express the insulin receptor under basal conditions, but once activated, they upregulate this receptor, showing insulin sensitivity [38]. Therefore, it is not yet clear how hyperinsulinaemia and insulin resistance influence the immune response.
Over the years, the effect of excess glucose and fatty acids, typical of obesity, on the immune balance has also been studied. Several immune cells express surface glucose transporters (GLUTs), such as GLUT1 and GLUT3, which are responsible for the internalization of glucose necessary for cellular metabolism. Excessive exposure to this energy source results in an increase in ROS synthesis and lipid peroxidation [39]. The influence of fatty acids on the immune system has been studied in reference to their similarity to lipopolysaccharide (LPS); therefore, the ability to stimulate the Toll-like receptors present on T cells has been investigated [40]. Unfortunately, there has not yet been unanimous consensus, and further studies are expected to analyse the role of fatty acids in regulating the immune response.
Considering the above, excess adipose tissue violates the well-balanced system of adipocytes and immune cells, with subsequent disturbances in the immune surveillance system, both innate and adaptive [28]. Regarding innate immunity, obesity is accompanied by altered neutrophil function, increased proinflammatory M1 macrophage activity, abnormal natural killer (NK) cell phenotypes, and an increased inflammatory response of dendritic cells, resulting in an overall altered first line of defence, increased inflammatory response, and abnormal T-cell response, as seen in a low-grade chronic systemic mechanism [41]. Under conditions of excess weight, the number of macrophages in the adipose tissue increases for both local proliferation of resident macrophages and the recruitment of peripheral monocytes. Furthermore, dysfunctional adipocytes in subjects with obesity produce proinflammatory cytokines that favour the activation of M1 macrophages despite M2 macrophages. Hyperglycaemia, frequently encountered in obesity, also appears to favour the proinflammatory polarization of macrophages [42].
Neutrophil function was studied in a case-control study in which 18 subjects with severe obesity (BMI: 35–68 kg/m2) were matched with 14 normal weight subjects. In the subjects with obesity, neutrophils retained phagocytosis ability and showed significant elevation in the release of basal superoxide (P < 0.0001) levels and chemotactic (P < 0.0003) and random (P < 0.0001) migration compared with lean controls. Therefore, the ability of neutrophils to fight infections was maintained in patients with excess weight, but oxidative stress associated with obesity was sustained by the overproduction of superoxide by neutrophils [43]. Furthermore, a study conducted in mice showed that a high-fat diet induced the upregulation of ligands of the NK cell-activating receptor (NCR1) on adipocytes of visceral adipose tissue. The induced NK cell proliferation and interferon-g (IFN-g) production, which in turn triggered the differentiation of proinflammatory macrophages, was considered a further stimulus for the polarization of M1 macrophages [44]. The innate immune response represents the first line of defence and coordinates the adaptive immune response and is particularly important in the response process to viral and bacterial infections; therefore, the alterations occurring in subjects with obesity are claimed to be responsible for greater susceptibility to infections [41]. Additionally, the adaptive immune response is compromised in obesity, resulting in decreased γδ T-cell functions, increased inflammatory T helper phenotypes, decreased regulatory T cells, and impaired B-cell functions, which inevitably leads to a less effective response against viral and fungal infectious agents and therefore causes a higher incidence and more severe disease course in subjects with obesity [41].
In a recent study, BMI was found to be inversely related to the number of γδ T cells [45]. These cells play a crucial function in wound repair, so their dysfunction can lead to delays in wound healing and infectious complications. A proinflammatory state in individuals with obesity is also due to the increase in Th17 and Th22 lymphocytes [46]; although the evidence is still limited, this process boosts the cascade process that can lead to a worsening of chronic systemic inflammation and worsening of the host response to infections. Moreover, Tregs seem to be decreased in patients with obesity, as observed by Wagner et al., who demonstrated reduced circulating Treg cell numbers in the blood of the obese group (BMI ≥ 27 kg/m2) compared with the nonobese group (BMI < 27 kg/m2) [47]. This subpopulation of T lymphocytes is specialized in suppressing the activation of the immune system towards autoantigens and thus in maintaining tolerance to self, thus excess weight could also affect the regulation of autoimmunity. An interesting aspect in support of the obesity-related nature of these alterations is that some of them can be reversed following bariatric surgery, suggesting the role of weight loss in the homoeostasis of the immune system [48].
In the context of alterations in immune system cells, although there is very limited evidence, differences between the conditions of metabolically healthy and metabolically unhealthy obesity have been detected. In particular, in a recent study, ten metabolically healthy subjects with obesity (Edmonton Obesity Staging System (EOSS) stage 0) and nine subjects with obesity and T2DM (EOSS stage 2) matched for BMI were evaluated. Subjects with stage 2 obesity had higher proportions of cytotoxic T cells, activated helper T cells (CD4+CD278+), and inflammatory monocytes (CD14+CRTh2+) and increased production of ROS by activated neutrophils than metabolically healthy subjects with obesity [49]. This finding suggested that metabolically unhealthy individuals with obesity and T2DM have impaired neutrophil function and T-cell responses, which may be partly responsible for the increased prevalence of infection susceptibility. Another study showed that the absolute count of proinflammatory monocytes (Mon2A, Mon3A) was lower in metabolically healthy obese individuals than in metabolically unhealthy individuals, although it was higher than that in healthy lean controls. Again, this suggests that some immune alterations, i.e., the presence of low-grade inflammation, even if not clinically apparent, is present in cases of obesity without metabolic complications and cannot be considered a benign condition in any case [50]. Differences in the immune response between metabolically healthy or unhealthy obese individuals could lead to a different susceptibility to infection or to different severity degrees of prognosis; however, in the literature, there are not yet adequate epidemiological studies comparing the two populations in this regard.
Vitamin D deficiency
Another common condition in obesity, which is potentially linked to infection susceptibility, is the deficiency of vitamins and, in particular, vitamin D; it has been reported that vitamin D deficiency increases the predisposition to systemic infections and impairs the immune response [51,52,53,54]. Both the National Health and Nutrition Examination Survey III (https://www.cdc.gov/nchs/nhanes/index.htm) and the Framingham study (https://framinghamheartstudy.org/) demonstrated a linear correlation between BMI and vitamin D values. A recent meta-analysis revealed that the prevalence of hypovitaminosis D in the population with obesity, regardless of age, latitude, cut-offs used to define the vitamin status and socioeconomic development index of the analysed geographical area, was 35% greater than normal weight and 24% higher than overweight [55]. One possible explanation for this phenomenon is that receptors for vitamin D are expressed in a conspicuous number of organs and tissues, including visceral and subcutaneous adipose tissue, both in human differentiated adipocytes (white and brown) and in cell cultures of preadipocytes [52]. Thus, once absorbed, vitamin D is seized and stored in different tissues, mainly in adipose tissue, as well as muscle tissue [56]. Additionally, the cells of both the innate and acquired immune systems express the vitamin D receptor (VDR) [52]. In vitro studies have observed that CYP27B1, an enzyme that activates vitamin D, is expressed within both monocytes/macrophages and dendritic cells (DCs) [52]. The immunomodulatory activity of vitamin D takes place within monocytes/macrophages by stimulating the synthesis of cathelecidine, a protein with antibacterial activity, and other cytokines, such as IL-1beta and IL-8 [57], while in DCs, it inhibits their maturation and therefore their ability to present the antigen in an infectious process [57]. Kuo et al. observed that there was no increase in TNFα levels when monocytes pretreated with 1,25(OH)2D3 were exposed to LPS [58]; likewise, another in vitro study demonstrated that preactivated human monocytes with LPS, once exposed to 1,25(OH)2D3, did not produce IL1α, IL6 and TNF-α in a dose-dependent fashion [59].
Moreover, vitamin D is also involved in the regulation of the adaptive immune system. In particular, it has been observed that the expression of VDR on T and B lymphocytes in resting conditions is low, while in conditions of activation and proliferation, these cells upregulate the expression of VDR, which, in turn, regulates the downstream transcription of more than 500 genes involved in the differentiation and proliferation of T and B lymphocytes [60]. Several in vitro studies have observed that calcitriol stimulation results in an anti-inflammatory polarization of lymphocytes, inhibiting the Th1 response in favour of a Th2 response: the production of IL2, IL6 and IL17 decreases in favour of the production of IL10 when CD4 T cells are treated with 1,25(OH)2D3 [61, 62].
Since vitamin D acts to suppress T-cell-driven inflammation and enhance the effects of suppressive Treg cells, its deficiency could be crucial in the pathogenesis of both infectious and autoimmune diseases [57]. In contrast, vitamin D supplementation can prevent respiratory infections through several immunoregulatory functions, including the reduced production of proinflammatory cytokines by the innate immune system [63]. For upper respiratory tract infections (RTIs), in the National Health and Nutrition Examination Survey, in a cohort study of 18,883 participants, it was observed that concentrations of 25(OH) vitamin D were independently and inversely associated with recent infections: subjects with circulating levels <10 ng/L presented a 1.4 times higher likelihood of developing infectious diseases than those with 25(OH) vitamin D levels >30 ng/L [64].
Cofactors
Pulmonary physiology is significantly modified by excess weight, which provokes reduced lung volumes, decreased compliance, abnormal ventilation and perfusion relationships, and respiratory muscle inefficiency [65]. Obesity causes a reduction in functional residual capacity (FRC) and expiratory reserve volume (ERV), with an inverse relationship between BMI and FRC [65]. In addition, excess weight also determines a reduction in lung and chest wall compliance, which has been shown to be exponentially related to BMI [65]. This depends both on decreased volumes, causing collapse and atelectasis of the smaller airways and an increase in alveolar surface tension, and on chest wall overload linked to adipose accumulation around the ribs, diaphragm, and abdomen, which can worsen respiratory mechanics, especially in the supine position [65]. Obesity has also been associated with ventilation/perfusion (V/Q) mismatching [66]. In fact, unlike healthy subjects, patients with obesity might present hypoventilation in the lower zones [66]. This depends on both small airway closure caused by lower ERV and reduced chest wall compliance, which can induce V/Q mismatching and hypoxaemia. Moreover, excess weight leads to greater fatigue of the respiratory muscles and increased oxygen consumption by those muscles, further worsening respiration [67]. Patients with obesity also have additional critical care limitations to consider, including difficulty in intubation, extubation, mask ventilation, prone positioning, and higher ventilatory pressures required [68]. Thus, alterations in respiratory mechanics and physiology can be responsible for an increased risk of pulmonary infection and contribute to a worse prognosis [69]. Pulmonary consolidation during pneumonia can cause an intrapulmonary shunt and a varying degree of V/Q mismatch, which can cause V/Q alterations, as reported in subjects with obesity, and can exacerbate hypoxemic respiratory failure [70]. Although no skin infection is specifically linked to obesity, some, including candidiasis, intertrigo, folliculitis, furunculosis, erythrasma, tinea cruris and erysipelas, have a higher incidence in subjects with obesity [71, 72]. Excess weight predisposes patients to lymphedema, alterations in micro- and macrocirculation with essential development of obesity-related micro- and macroangiopathy [73] and delayed wound healing [69, 74, 75]. Obesity worsens lymphatic flow, involving a collection of protein-rich lymphatic fluid in the subcutaneous tissue, which can lead to lymphedema [76]. Obesity also causes impaired capillary recruitment and a significant reduction in the cutaneous vasomotor response to autonomic activation [73]. This progressive accumulation of fluid results in reduced tissue oxygenation, which can cause fibrosis and a chronic inflammatory state over time [76]. Furthermore, lymphedema’s disruption of immune cell trafficking leads to localized immune suppression, with the development of chronic inflammation and infections such as cellulitis and verrucosis [74], contributing to secondary complications in the lower extremities, such as foot infections and ulcerations [77]. In an observational study, two cohorts of patients with lymphedema confirmed by lymphoscintigraphy were compared: Group 1, normal weight patients (BMI ≤ 25 kg/m2) and Group 2, patients with obesity (BMI ≥ 30 kg/m2) [41]. Group 2 was more likely to undergo infection (59%, P < 0.001) and hospitalization (47%, P < 0.001) than Group 1 [75]. The presence of infection can cause delayed wound healing, increased hospitalization, increased health-care costs and reduced patient quality of life [78], which boosts the vicious circle of obesity, skin infections and delayed wound healing.
Obesity is associated with a number of comorbidities constituting metabolic syndrome, such as T2DM, hypertension and CVD, already known as predictive factors of susceptibility to infections [79,80,81]. It has been observed that The Middle East respiratory syndrome coronavirus (MERS-CoV) is associated with life-threatening severe illnesses and a mortality rate of approximately 35%, especially in patients with these underlying comorbidities [79]. In particular, the presence of T2DM leads to an increase in the incidence of respiratory infections and is often identified as an independent risk factor for developing lower RTIs [80]. While respiratory infections by Mycobacterium tuberculosis, Staphylococcus aureus, gram-negative bacteria and fungi are more common in the general population, those from Streptococcus and the flu virus may be more associated with increased morbidity and mortality in subjects with T2DM [80]. Hospitalization due to the flu virus or flu-like infections is up to six times more likely to occur in patients with T2DM than in the healthy population, and diabetic patients are hospitalized after infection complications more frequently [80]. Furthermore, a meta-analysis on more than 30 million subjects highlighted not only that having a higher BMI significantly increased the risk of postoperative sepsis but also that several comorbidities, including T2DM, chronic kidney disease, heart failure, cerebrovascular disease and CVD, further augmented the risk of postoperative sepsis [81].
Another aspect that is often underestimated and can affect the prognosis of subjects with obesity and infections is the inappropriate treatment per weight [82]. The condition of obesity can modify the pharmacokinetics of drugs, depending on multiple factors, including the degree of obesity, organ functions and drug characteristics [82]. The drug distribution phase results are severely different because of both alteration of plasma protein binding and volume of distribution (VD) of drugs: VD of lipophilic drugs (fluoroquinolones) increases while that of hydrophilic drugs (amikacin and tobramycin) lows [82]. In a review board-approved retrospective study at the American Society of Metabolic and Bariatric Surgery Center of Excellence, data from the emergency department (ED) evaluating adherence to hospital guidelines in the first prescription of cefepime, cefazolin or ciprofloxacin to patients with obesity were collected, and it was observed that the adherence rates for the first dose of these drugs administered were 8.0%, 3.0%, and 1.2%, respectively; therefore, these antimicrobials were frequently underdosed in subjects with obesity [83].
In the following sections, infections will be analysed according to body region and the role of excess weight will be discussed (Fig. 2).
Several epidemiological studies have demonstrated that overweight and obesity are associated with a higher prevalence of respiratory tract infections (RTIs), characterized by a longer duration of disease and higher mortality. Upper infections (common cold, pharyngitis, otitis, sinusitis, laryngotracheitis and epiglottitis) and lower infections (bronchitis, pneumonia and bronchiolitis) have a higher prevalence among subjects with obesity and weight gain would seem to increase the risk of RTIs. Excess weight has been identified as a risk factor and recurrence factor for skin infections, in particular the incidence of onychomycosis and cellulitis increases in proportion to BMI. Candida Albicans infections are more prevalent among the population with obesity and also more complicated than normal weight populations. Erythrasma would seem to be complicated by the condition of overweight, probably, due to skin folds. Moreover, obesity is associated with a significantly increased risk of skin and soft-tissue infection when undergoing general, orthopaedic, gynaecological and bariatric surgery. Finally, several studies have identified obesity as risk factor for urinary tract infections, also in specific conditions such as admission in intensive care or moderate or major surgery, or after traumatic injury.
Skin infections
A higher incidence of cutaneous infections has been reported in patients with obesity than in nonobese patients (Table 2), which is probably due to changes in skin barrier function, lymphatic system, collagen structure and function, the latter being also responsible for wound-healing difficulties.
A dysfunction in the production process of senile adipocytes in the dermis is most likely the reason for the increased risk of infections in obesity. In a recent study, it was observed that a high-fat dietary regimen promotes hyperplastic growth of adipocytes, which complete their maturation process off dermal progenitors. These progenitors feature stronger capabilities of secretive antimicrobial peptides, and this peculiar function is lost in the maturation process [84]. The decrease in adipocyte progenitors in diet-induced obese (DIO) mice was explained by the expression of transforming growth factor–β (TGFβ) by mature adipocytes that then inhibited adipocyte progenitors and the production of cathelicidin, a powerful antimicrobial agent, in vitro [84]. It was also observed that administration of a TGFβ receptor inhibitor reversed this inhibition in both cultured adipocyte progenitors and in mice and subsequently restored the capacity of obese mice to defend against S. aureus skin infection [84]. Therefore, the increase in dermal mature adipocytes associated with obesity seems to weaken the immune response towards skin infections. Cellulitis has a high incidence in subjects with obesity. In particular, in a large cohort of 171,322 Korean adults followed from 2011 to 2016, increased BMI was associated with an increased risk of cellulitis and hospitalization in both metabolically healthy and unhealthy individuals, suggesting that obesity can be an independent risk factor for cellulitis regardless of the metabolic phenotype [71]. In addition, severe cellulitis tends to occur more commonly in the legs of patients with coexisting lymphedema, a condition frequently encountered in subjects with obesity [85].
Several studies have indicated that obesity predisposes individuals to erysipelas independently of potential confounders [21, 72], and it is a known risk factor for its complications [86, 87]. In a retrospective chart review of patients hospitalized for primary and recurrent erysipelas, it was found that obesity prolongs the time of hospitalization [86, 87], while another study showed that obesity is associated with erysipela recurrence notwithstanding prophylactic treatment with benzathine penicillin G [87]. Candida albicans infection is also more prevalent in patients with obesity and might cause folliculitis, intertrigo, furunculosis, or paronychia of the hands or feet [88]. The presence of skin folds such as inframammary, genitocrural, axillary and abdominal folds, which favour macerated erythaematous plaques along with eventual poor local hygiene, are predisposing factors for the onset of cutaneous mycosis, especially recurrent Candida intertrigo [89].
Obesity is also one of the most important risk factors for onychomycosis [90, 91]. Significant increases in the incidence of onychomycosis have also been observed in inpatient clinic attendees with obesity [90]. A Chinese study conducted on 877 adult patients revealed that obesity is one of the three most prevalent predisposing factors (vascular disease and diabetes are the other two) for fungal nail disease [91]. Furthermore, skin infections appear to be associated with excess weight even in children [92]. In a retrospective, population-based study at the Kaiser Permanente Northern California Managed Health care System, the presence of skin disorders in 248,775 children was assessed, and childhood obesity was observed to be associated with a higher prevalence of bacterial and fungal skin infections [92].
Urinary tract infections
UTIs are one of the most commonly acquired bacterial infections in outpatient and hospitalized populations; its prevalence is approximately 11% in the overall population [93]. Women are more affected than men [94], and they also present a greater risk of recurrence [95]. Several studies have identified an association between UTIs and obesity (Table 3). In a descriptive study on 95,598 subjects, Semins et al. demonstrated an association between obesity and both UTIs and pyelonephritis, with a higher prevalence in female patients [96]. In line with these observations, in a prospective cohort study on 457 patients with T2DM, obesity was found to be a risk factor for UTIs in a male population [97]. Some studies have shown that obesity can be a risk factor for UTIs in specific conditions, such as admission to intensive care, moderate or major surgery, or after traumatic injury [3, 4, 98]. In a prospective study on 1105 patients admitted to the ICU over a 2-year period, mostly due to trauma, a twofold increase in the relative risk of acquiring a UTI was observed in patients with obesity [7]. Furthermore, a high BMI was independently associated with a higher rate of ICU-acquired UTIs (P = 0.02) in a retrospective study on 301 patients affected by septic shock [8]. Patients with obesity had a significantly higher risk of UTIs than nonobese patients, and obesity was found to be an overall independent risk factor for perioperative morbidity [98]. It was also observed that obesity was associated with UTIs regardless of the presence of comorbidities such as type T2DM or vitamin D deficiency [99]. Regarding the gender difference in UTIs, the recent Danish Donor Blood study has shown that UTIs are not only more common in females [100] but also specifically during pregnancy and the postpartum period [9, 10]. Obesity was a risk factor for UTI in 767 pregnant women [9] and in 8350 postpartum women in a population‐based observational study [10], and again, it determined a worse prognosis and course of UTI [9, 10]. A case−control study on premenopausal non-pregnant women with recurrent UTI (RUTI), defined as symptomatic UTI that follows the resolution of a previous UTI or three or more symptomatic episodes over a 12-month period, demonstrated that the mean BMI among women with RUTIs was significantly higher than controls [101], suggesting that obesity may also have a role in recurrent forms, which are frequent in women. Several paediatric studies have analysed the association between obesity and UTIs [102, 103]. In a retrospective case−control study on 41,819 patients aged 2−20 years, obesity was strongly associated with the presence of UTIs in the female paediatric hospitalized population, and the risk of UTIs was increased by 45% in females with obesity [102]. Therefore, also in paediatric patients with obesity, the management of body excess weight and urinalysis should be considered as early as possible, considering the higher incidence of UTIs in overweight and obese children <2 years of age presenting with fever compared to normal weight febrile children [103]. Moreover, the negative prognostic value of obesity was also confirmed in subjects with UTIs aged <18 years [104].
Regarding the pathophysiological mechanisms, a common unanimous consensus has not yet been achieved. For example, glycosuria represents a predisposing factor for UTIs in subjects with diabetes. However, not all diabetic subjects have glycosuria, which does not explain the increased incidence of UTIs in nondiabetic subjects with obesity, suggesting the existence of other mechanisms. The urinary tract constitutively expresses antimicrobial agents such as several nonenzymatic ribonucleases (RNases), lipocalin 2 (Lcn2), defensins and cathelicidin LL-37, whose secretion can also be induced by higher levels of bacteria in the urine [105]. These act mainly by preventing microbes from adhering to the epithelium or by preventing their replication. It has been shown that the expression of some antimicrobial peptides, particularly RNase7, by the uroepithelium could be induced by insulin via the classical insulin signalling pathway. In particular, using primary human urothelial cells, it was demonstrated that insulin induces RNase 7 production via the phosphatidylinositide 3-kinase signalling pathway (PI3K/AKT) to shield urothelial cells from uropathogenic E. coli [106]. Evaluating obese hyperglycaemic db/db mice, which exhibit a T2DM phenotype, an increased susceptibility to uropathogenic E. coli inoculation was observed [107]. Moreover, insulin-resistant normoglycaemic TALLYHO mice had increased UTI susceptibility, independent of hyperglycaemia or glucosuria, suggesting a role for insulin resistance more than glycosuria in this process [107]. Furthermore, using murine and human primary renal epithelial cells, Murtha et al. demonstrated that RNase4 and Lcn2 from both sources were induced by the presence of insulin and that the response could be suppressed by administration of a PI3K inhibitor, wortmannin, supporting the role of classical insulin signalling in the induction of antimicrobial agents and the plausible role of insulin resistance in increased susceptibility to UTIs [107]. On the other hand, it is not yet known whether the link between insulin resistance and UTIs can be mediated by an excess of generalized or predominantly kidney-localized visceral adipose tissue. One study has shown that the presence of perinephric fat stranding is an independent predictive factor for febrile UTIs after ureteroscopic lithotripsy [108], suggesting a role of perirenal fat in the onset of UTIs.
Respiratory tract infections
It is well known that obesity is associated with an increased risk of RTIs (Tables 1 and 4) [100]. As mentioned before, a chronic proinflammatory state in association with an excessive oxidative stress response and impaired immunity (typical of overweight/obese patients) could explain this relationship [28, 29, 73]. Obesity could be considered either a risk factor for the onset of RTIs or as a risk factor for poor prognosis [67]. There is no evidence that excess adipose tissue at the level of the lungs can be a determinant for the risk of respiratory infections. The accumulation of fat at the abdominal visceral level seems to be a negative prognostic factor for patients suffering from severe pulmonary deficiency, as in SARS-CoV-2 disease (COVID-19). It has been observed that for every 1-cm increase in abdominal circumference in COVID-19 patients, the risk of intensive care admission increased by 1.13 and for mechanical ventilation by 1.25 [109]. Similarly, excess fat at the level of the neck is related to a worse prognosis: a neck circumference greater than 42.5 cm in men/37.5 cm in women was associated with early intubation in COVID-19 patients compared to patients with smaller circumferences [110]. These observations could be justified both by mechanical reasons, causing a reduction in airflow in the upper airways or a reduction in the expansion of the rib cage, and by paracrine proinflammatory mechanisms of adipose tissue that could modify the immune response. Moreover, the correlation between the risk of infection and severe course of COVID-19 and obesity has been investigated in relation to the expression of angiotensin converting enzyme-2 (ACE2), considering that SARS-CoV-2 interacts with ACE2 to enter alveolar cells involving the serine protease TMPRSS2. Al Heialy et al. showed that in obese mice, there was an upregulation of ACE2 and TMPRSS2 PMC [111]; this observation, however, has not been confirmed by studies conducted in humans, in which there would be no significant variations in the expression of ACE2 in adipose tissue of obese vs. nonobese patients [112]. Human studies have also evaluated the change in ACE2 expression following weight reduction, and while some studies have found a reduction in ACE2 expression, others have not identified any significant difference, so there are no consistent results in the literature to link obesity-ACE2 expression and the risk of SARS-CoV-2 infection [113, 114]. Therefore, in subjects with obesity, the role of ACE2 in the pathophysiology of COVID-19 remains controversial. It is important to consider that excess weight is associated with an environment that favours vascular damage, inflammation, remodelling of membranes and subsequent alteration of vascular flow with risk of hypoxia and development of thrombosis [115]. Several epidemiological studies have demonstrated that overweight and obesity are associated with a higher prevalence of RTIs, characterized by a longer duration of disease and higher mortality [3, 4, 116,117,118,119,120,121,122,123,124]. Obesity impairs the immune response to flu and flu vaccination through alterations of the cellular immune system, probably due to hyperinsulinaemia or hyperleptinemia that could dysregulate T-cell metabolism [48]. During the first pandemic influenza 2009/H1N1 swine flu, obesity was identified as an independent risk factor for increased morbidity and mortality based on public health surveillance data of hospitalized California residents [116]. A case−control study has shown that morbid obesity (BMI ≥ 40) was an independent risk factor for hospitalization among 564 hospitalized adult patients with H1N1 flu, with or without recognized chronic conditions (cardiovascular disease, pulmonary disease, liver disease, cancer, and diabetes) [117]; in contrast, underweight patients 2−19 years old had a higher hospitalization rate than patients with obesity [117]. This negative role of obesity was confirmed by two Spanish prospective, observational, multicentric studies that identified a longer hospitalization and a more frequent and longer use of mechanical ventilation among H1N1 patients with obesity, albeit similar death rates [125, 126]. In addition, the WHO conducted a study to evaluate risk factors associated with H1N1 prognosis among 20 countries, and it was found that obesity, asthma and pregnancy were related to disease severity [3]. Some studies did not identify the same association: obesity was not related to any flu viruses among adults ≥20 years with a medical encounter for acute respiratory illness [4, 127]. These contrasting results make it difficult to understand the actual effect of obesity on susceptibility to influenza and the utility of starting an antiviral therapy protocol sooner than nonobese patients. Nevertheless, the association between obesity and RTIs may not be limited to the flu season [118]: a retrospective cohort study of 13 years on 104,665 individuals in Ontario demonstrated that subjects with obesity were at an increased risk of outpatient visits for RTIs during both flu and nonflu periods [118]. This might occur as a consequence of obesity-related enhanced susceptibility to any viral and bacterial respiratory pathogens [118]. BMI has also been evaluated as a risk factor for other respiratory infections: a recent prospective study conducted on a large cohort of 1455 patients showed that patients who were overweight and obese had a higher prevalence of RTIs, considering both upper infections (common cold, pharyngitis, otitis, sinusitis, laryngotracheitis, epiglottitis) and lower infections (bronchitis, pneumonia and bronchiolitis) [122]. In particular, lower infections were more common among obesity-affected patients, and the association between pneumonia and bronchitis was statistically significant [122]. In the Canadian Healthy Project, BMI was related to an increase in the self-report diagnosis rate of chronic bronchitis after exposure to a second risk factor, such as smoking [124]. Additionally, in the paediatric population (6−17 years), obesity has been identified as a risk factor for chronic bronchitis [120, 121]. The association between BMI and RTIs suggests that their incidence could be possibly decreased by lifestyle interventions [119]. A study found that weight gain in the male population had a twofold increase in the likelihood of community-acquired pneumonia (CAP) compared with those who maintained their weight, but a linear correlation was not found between BMI and CAP in women [119]. More data are needed to understand sex differences in the relation between obesity and the risk of pneumonia [119]. A recent retrospective study conducted on paediatric inpatients aged between 2 and 20 years with pneumonia or bronchitis showed that obesity was significantly associated with the use of both noninvasive and invasive mechanical ventilation and with the development of septicaemia/bacteremia [123]. Although the relationship between obesity and RTIs appears cogent, further studies are needed to confirm it.
Surgical site infections
SSIs are postoperative infections that occur within 30 days of a surgical procedure (or within 1 year for permanent implants) and constitute up to 19.6% of hospital-acquired infections (HAIs), as was revealed in a survey of health-care-associated infections and antimicrobial use in European acute care hospitals. SSIs negatively impact patient health by increasing both morbidity and mortality [128] and decreasing overall patient quality of life [129] as well as the public health economy, causing prolonged hospitalization with an additional cost of management [130]. Previous prospective cohort studies have indicated that obesity is associated with a significantly increased risk of skin and soft-tissue infections after surgery [14,15,16].
T2DM has been shown to be an independent risk factor for SSIs, and considering its frequency in patients with obesity, it should be carefully investigated and treated before any surgical procedure [15]. One of the reasons for the greater risk of surgical site infections in patients with obesity is difficulties in wound healing, which promote the entrance and proliferation of microorganisms. It is known that the pathological expansion of white adipose tissue in conditions of excess weight modifies blood flow; indeed, there is cross-talk between endothelial cells and adipocytes that regulates the production of nitric oxide and therefore vasodilation [131]. Several studies have shown less endothelium-induced vasodilation in association with excess weight [132]; hence, deceased blood flow could cause unfavourable wound healing. In addition, the influence of adipose tissue on γδ T-cell activity alters their auxiliary function in tissue repair [45].
Waisbren et al. carried out a prospective study that included 591 adult elective surgical patients evaluated during preoperative, operative, and 30-day postoperative periods, and they found that patients with obesity, defined by body fat percentage (%BF) > 25% in men and >31% in women, at a bioelectrical impedance analysis were five times more likely to have SSIs after surgery [14]. In contrast, using BMI > 30 kg/m2 as a diagnostic parameter of obesity, there was no significant difference in the incidence of SSIs between subjects with or without obesity [14]. Given these results, they concluded that BF% was a more sensitive and precise measurement than BMI in determining the association between obesity and the risk of postoperative SSIs [14]. Hence, more studies should be conducted using %BF instead of BMI, although it is more difficult to calculate. The main studies on SSIs and obesity are summarized in Table 5.
Bariatric surgery
Bariatric surgery has become a very effective option to treat obesity, providing adequate and effective weight loss, improving quality of life and reducing morbidity and mortality [133], albeit it requires appropriate pre- and postsurgical adherence to dietary attitudes and eventual medical treatment [134]. Particular attention is necessary to manage the complications of surgery in patients with obesity, especially the risk of SSIs. A retrospective study that compared two different groups with obesity, a morbidly obese group (BMI 35–49 kg/m2) and a superobese group (BMI ≥ 50 kg/m2), showed that the latter had a significantly greater incidence of postoperative complications, including superficial and deep wound infections, sepsis, septic shock and increased 30-day mortality [135]. A recent study that analysed 334 patients who underwent open bariatric surgery and 262 patients who underwent video-laparoscopic bariatric surgery from July 2008 to January 2018 showed that a higher body mass index was associated with a higher incidence of SSIs, despite the use of a dedicated preventive strategy to correct risk factors [136]. Moreover, the laparotomy approach and the presence of T2DM increased the rate of SSIs [136]. Accordingly, in patients undergoing bariatric surgery, it is recommended to reduce the modifiable risk factors for SSIs (weight excess and T2DM) and adopt a laparoscopic approach.
General surgery
The association between SSIs and obesity results in the decreased oxygen tension of the relatively avascular adipose tissue, differences in wound healing, greater wound size, or technical difficulties [35,36,37,38]. SSIs are the most common complication after colectomy, and obesity can increase this risk by 2.5−5 times [137]. Obesity is an established risk factor for the development of colorectal cancer; hence, many studies can be found regarding the risk stratification of colorectal cancer surgery complications [138]. A recent retrospective study on a population of 74,891 subjects with colorectal cancer stratified according to BMI showed that patients with obesity experienced incremental odds of SSIs after elective colorectal surgery as BMI increased [139]. Similarly, an observational study on 3202 patients who underwent colectomy for cancer showed that patients with morbid obesity (BMI > 35 kg/m2) had a higher risk of surgical site infection than normal weight patients, confirming a linear association between BMI and SSI incidence after colectomy for colorectal cancer [140]. In a retrospective study on 414 patients with colorectal cancer who underwent surgery, it was observed that postoperative pelvic abscesses were more common in patients with obesity than in nonobese patients [141]. Furthermore, even after liver resection surgery, obesity was found to be a risk factor with predictive value for SSI [142]. Gervaz et al. defined risk factors in the so-called COLA score (contamination, obesity, laparotomy and American Society of Anaesthesiologists grade—ASA). They demonstrated that obesity, contamination class 3–4, ASA grade III–IV and open surgery significantly increased the risk for SSIs [143]. BMI was found to be an independent factor for the occurrence of serious postoperative infectious complications in patients who underwent pancreatoduodenectomy [144, 145]. Furthermore, a prospective 6-year cohort study showed that obesity is a risk factor for SSIs in cardiac surgery, especially in valve surgery [146], in which sternal wound infection, mediastinitis and bacteremia are the most common infectious complications [146, 147]. Obesity has also been linked with other types of surgery, such as orthopaedic [148] and gynaecologic (caesarean) surgery [116, 149], showing a strong relationship between excess adiposity and SSIs.
Conclusions
Subjects with obesity definitively present a higher risk of contracting different infectious diseases, as well as experiencing a more severe course with increased mortality rates. Considering the increasing rate of obesity worldwide, it is necessary to investigate possible mechanisms and processes that underlie this association to improve preventive and therapeutic strategies. Furthermore, it is expected that this higher infectious risk along with cardiovascular mortality can provide a strong incentive for weight loss in subjects with obesity. Campaigns for the prevention of excess weight can be useful tools to promote a healthy lifestyle with a goal to decrease the rates of obesity and related complications.
References
Huttunen R, Syrjanen J. Obesity and the risk and outcome of infection. Int J Obes (Lond). 2013;37:333–40.
Fezeu L, Julia C, Henegar A, Bitu J, Hu FB, Grobbee DE, et al. Obesity is associated with higher risk of intensive care unit admission and death in influenza A (H1N1) patients: a systematic review and meta-analysis. Obes Rev. 2011;12:653–9.
Van Kerkhove MD, Vandemaele KA, Shinde V, Jaramillo-Gutierrez G, Koukounari A, Donnelly CA, et al. Risk factors for severe outcomes following 2009 influenza A (H1N1) infection: a global pooled analysis. PLoS Med. 2011;8:e1001053.
Murphy R, Fragaszy EB, Hayward AC, Warren-Gash C. Investigating obesity as a risk factor for influenza-like illness during the 2009 H1N1 influenza pandemic using the Health Survey for England. Influenza Other Respir Viruses. 2017;11:66–73.
Kim CO, Nam CM, Lee DC, Chang J, Lee JW. Is abdominal obesity associated with the 2009 influenza A (H1N1) pandemic in Korean school-aged children? Influenza Other Respir Viruses. 2012;6:313–7.
Bijani B, Pahlevan AA, Qasemi-Barqi R, Jahanihashemi H. Metabolic syndrome as an independent risk factor of hypoxaemia in influenza A (H1N1) 2009 pandemic. Infez Med. 2016;24:123–30.
Bochicchio GV, Joshi M, Bochicchio K, Nehman S, Tracy JK, Scalea TM. Impact of obesity in the critically ill trauma patient: a prospective study. J Am Coll Surg. 2006;203:533–8.
Wurzinger B, Dunser MW, Wohlmuth C, Deutinger MC, Ulmer H, Torgersen C, et al. The association between body-mass index and patient outcome in septic shock: a retrospective cohort study. Wien Klin Wochenschr. 2010;122:31–6.
Basu JK, Jeketera CM, Basu D. Obesity and its outcomes among pregnant South African women. Int J Gynaecol Obstet. 2010;110:101–4.
Usha Kiran TS, Hemmadi S, Bethel J, Evans J. Outcome of pregnancy in a woman with an increased body mass index. BJOG. 2005;112:768–72.
Choban PS, Heckler R, Burge JC, Flancbaum L. Increased incidence of nosocomial infections in obese surgical patients. Am Surg. 1995;61:1001–5.
Kaye KS, Marchaim D, Chen TY, Chopra T, Anderson DJ, Choi Y, et al. Predictors of nosocomial bloodstream infections in older adults. J Am Geriatr Soc. 2011;59:622–7.
Olsen MA, Higham-Kessler J, Yokoe DS, Butler AM, Vostok J, Stevenson KB, et al. Developing a risk stratification model for surgical site infection after abdominal hysterectomy. Infect Control Hosp Epidemiol. 2009;30:1077–83.
Waisbren E, Rosen H, Bader AM, Lipsitz SR, Rogers SO Jr., Eriksson E. Percent body fat and prediction of surgical site infection. J Am Coll Surg. 2010;210:381–9.
Di Leo A, Piffer S, Ricci F, Manzi A, Poggi E, Porretto V, et al. Surgical site infections in an Italian surgical ward: a prospective study. Surg Infect (Larchmt). 2009;10:533–8.
Beldi G, Bisch-Knaden S, Banz V, Muhlemann K, Candinas D. Impact of intraoperative behavior on surgical site infections. Am J Surg. 2009;198:157–62.
Towfigh S, Chen F, Katkhouda N, Kelso R, Sohn H, Berne TV, et al. Obesity should not influence the management of appendicitis. Surg Endosc. 2008;22:2601–5.
Zahr F, Genovese E, Mathier M, Shullo M, Lockard K, Zomak R, et al. Obese patients and mechanical circulatory support: weight loss, adverse events, and outcomes. Ann Thorac Surg. 2011;92:1420–6.
Torgerson JS, Lindroos AK, Naslund I, Peltonen M. Gallstones, gallbladder disease, and pancreatitis: cross-sectional and 2-year data from the Swedish Obese Subjects (SOS) and SOS reference studies. Am J Gastroenterol. 2003;98:1032–41.
Martinez J, Johnson CD, Sanchez-Paya J, de Madaria E, Robles-Diaz G, Perez-Mateo M. Obesity is a definitive risk factor of severity and mortality in acute pancreatitis: an updated meta-analysis. Pancreatology. 2006;6:206–9.
Karppelin M, Siljander T, Vuopio-Varkila J, Kere J, Huhtala H, Vuento R, et al. Factors predisposing to acute and recurrent bacterial non-necrotizing cellulitis in hospitalized patients: a prospective case-control study. Clin Microbiol Infect. 2010;16:729–34.
Bartholomeeusen S, Vandenbroucke J, Truyers C, Buntinx F. Epidemiology and comorbidity of erysipelas in primary care. Dermatology. 2007;215:118–22.
Carratala J, Roson B, Fernandez-Sabe N, Shaw E, del Rio O, Rivera A, et al. Factors associated with complications and mortality in adult patients hospitalized for infectious cellulitis. Eur J Clin Microbiol Infect Dis. 2003;22:151–7.
Lo Iacono O, Venezia G, Petta S, Mineo C, De Lisi S, Di, Marco V, et al. The impact of insulin resistance, serum adipocytokines and visceral obesity on steatosis and fibrosis in patients with chronic hepatitis C. Aliment Pharmacol Ther. 2007;25:1181–91.
Harpsoe MC, Nielsen NM, Friis-Moller N, Andersson M, Wohlfahrt J, Linneberg A, et al. Body mass index and risk of infections among women in the Danish National Birth Cohort. Am J Epidemiol. 2016;183:1008–17.
Phung DT, Wang Z, Rutherford S, Huang C, Chu C. Body mass index and risk of pneumonia: a systematic review and meta-analysis. Obes Rev. 2013;14:839–57.
Dorner TE, Schwarz F, Kranz A, Freidl W, Rieder A, Gisinger C. Body mass index and the risk of infections in institutionalised geriatric patients. Br J Nutr. 2010;103:1830–5.
Nave H, Beutel G, Kielstein JT. Obesity-related immunodeficiency in patients with pandemic influenza H1N1. Lancet Infect Dis. 2011;11:14–5.
Muscogiuri G, Pugliese G, Laudisio D, Castellucci B, Barrea L, Savastano S, et al. The impact of obesity on immune response to infection: plausible mechanisms and outcomes. Obes Rev. 2021;22:e13216.
Tilg H, Moschen AR. Adipocytokines: mediators linking adipose tissue, inflammation and immunity. Nat Rev Immunol. 2006;6:772–83.
Ziegler-Heitbrock HW, Wedel A, Schraut W, Strobel M, Wendelgass P, Sternsdorf T, et al. Tolerance to lipopolysaccharide involves mobilization of nuclear factor kappa B with predominance of p50 homodimers. J Biol Chem. 1994;269:17001–4.
Lord GM, Matarese G, Howard JK, Baker RJ, Bloom SR, Lechler RI. Leptin modulates the T-cell immune response and reverses starvation-induced immunosuppression. Nature. 1998;394:897–901.
Papathanassoglou E, El-Haschimi K, Li XC, Matarese G, Strom T, Mantzoros C. Leptin receptor expression and signaling in lymphocytes: kinetics during lymphocyte activation, role in lymphocyte survival, and response to high fat diet in mice. J Immunol. 2006;176:7745–52.
Caldefie-Chezet F, Poulin A, Tridon A, Sion B, Vasson MP. Leptin: a potential regulator of polymorphonuclear neutrophil bactericidal action? J Leukoc Biol. 2001;69:414–8.
Claycombe K, King LE, Fraker PJ. A role for leptin in sustaining lymphopoiesis and myelopoiesis. Proc Natl Acad Sci USA. 2008;105:2017–21.
Martin-Romero C, Santos-Alvarez J, Goberna R, Sanchez-Margalet V. Human leptin enhances activation and proliferation of human circulating T lymphocytes. Cell Immunol. 2000;199:15–24.
Trischitta V, Brunetti A, Chiavetta A, Benzi L, Papa V, Vigneri R. Defects in insulin-receptor internalization and processing in monocytes of obese subjects and obese NIDDM patients. Diabetes. 1989;38:1579–84.
Stentz FB, Kitabchi AE. Activated T lymphocytes in Type 2 diabetes: implications from in vitro studies. Curr Drug Targets. 2003;4:493–503.
Stentz FB, Kitabchi AE. Hyperglycemia-induced activation of human T-lymphocytes with de novo emergence of insulin receptors and generation of reactive oxygen species. Biochem Biophys Res Commun. 2005;335:491–5.
Lee JY, Sohn KH, Rhee SH, Hwang D. Saturated fatty acids, but not unsaturated fatty acids, induce the expression of cyclooxygenase-2 mediated through Toll-like receptor 4. J Biol Chem. 2001;276:16683–9.
Frydrych LM, Bian G, O’Lone DE, Ward PA, Delano MJ. Obesity and type 2 diabetes mellitus drive immune dysfunction, infection development, and sepsis mortality. J Leukoc Biol. 2018;104:525–34.
Torres-Castro I, Arroyo-Camarena UD, Martinez-Reyes CP, Gomez-Arauz AY, Duenas-Andrade Y, Hernandez-Ruiz J, et al. Human monocytes and macrophages undergo M1-type inflammatory polarization in response to high levels of glucose. Immunol Lett. 2016;176:81–9.
Brotfain E, Hadad N, Shapira Y, Avinoah E, Zlotnik A, Raichel L, et al. Neutrophil functions in morbidly obese subjects. Clin Exp Immunol. 2015;181:156–63.
Wensveen FM, Jelencic V, Valentic S, Sestan M, Wensveen TT, Theurich S, et al. NK cells link obesity-induced adipose stress to inflammation and insulin resistance. Nat Immunol. 2015;16:376–85.
Fay NS, Larson EC, Jameson JM. Chronic Inflammation and gammadelta T Cells. Front Immunol. 2016;7:210.
Xia C, Rao X, Zhong J. Role of T lymphocytes in Type 2 diabetes and diabetes-associated inflammation. J Diabetes Res. 2017;2017:6494795.
Wagner NM, Brandhorst G, Czepluch F, Lankeit M, Eberle C, Herzberg S, et al. Circulating regulatory T cells are reduced in obesity and may identify subjects at increased metabolic and cardiovascular risk. Obesity (Silver Spring). 2013;21:461–8.
Frikke-Schmidt H, Zamarron BF, O’Rourke RW, Sandoval DA, Lumeng CN, Seeley RJ. Weight loss independent changes in adipose tissue macrophage and T cell populations after sleeve gastrectomy in mice. Mol Metab. 2017;6:317–26.
Richard C, Wadowski M, Goruk S, Cameron L, Sharma AM, Field CJ. Individuals with obesity and type 2 diabetes have additional immune dysfunction compared with obese individuals who are metabolically healthy. BMJ Open Diabetes Res Care. 2017;5:e000379.
Christou KA, Christou GA, Karamoutsios A, Vartholomatos G, Gartzonika K, Tsatsoulis A, et al. Metabolically healthy obesity is characterized by a proinflammatory phenotype of circulating monocyte subsets. Metab Syndr Relat Disord. 2019;17:259–65.
Barrea L, Frias-Toral E, Pugliese G, Garcia-Velasquez E, DELAC M, Savastano S, et al. Vitamin D in obesity and obesity-related diseases: an overview. Minerva Endocrinol (Torino). 2021;46:177–92.
Bouillon R, Marcocci C, Carmeliet G, Bikle D, White JH, Dawson-Hughes B, et al. Skeletal and extraskeletal actions of vitamin D: current evidence and outstanding questions. Endocr Rev. 2019;40:1109–51.
Barrea L, Muscogiuri G, Frias-Toral E, Laudisio D, Pugliese G, Castellucci B, et al. Nutrition and immune system: from the Mediterranean diet to dietary supplementary through the microbiota. Crit Rev Food Sci Nutr. 2021;61:3066–90.
Muscogiuri G, Barrea L, Savastano S, Colao A. Nutritional recommendations for CoVID-19 quarantine. Eur J Clin Nutr. 2020;74:850–1.
Pereira-Santos M, Costa PR, Assis AM, Santos CA, Santos DB. Obesity and vitamin D deficiency: a systematic review and meta-analysis. Obes Rev. 2015;16:341–9.
Holick MF. Vitamin D deficiency. N Engl J Med. 2007;357:266–81.
Hewison M. An update on vitamin D and human immunity. Clin Endocrinol (Oxf). 2012;76:315–25.
Kuo YT, Kuo CH, Lam KP, Chu YT, Wang WL, Huang CH, et al. Effects of vitamin D3 on expression of tumor necrosis factor-alpha and chemokines by monocytes. J Food Sci. 2010;75:H200–4.
Muller K, Haahr PM, Diamant M, Rieneck K, Kharazmi A, Bendtzen K. 1,25-Dihydroxyvitamin D3 inhibits cytokine production by human blood monocytes at the post-transcriptional level. Cytokine. 1992;4:506–12.
Chen S, Sims GP, Chen XX, Gu YY, Chen S, Lipsky PE. Modulatory effects of 1,25-dihydroxyvitamin D3 on human B cell differentiation. J Immunol. 2007;179:1634–47.
Willheim M, Thien R, Schrattbauer K, Bajna E, Holub M, Gruber R, et al. Regulatory effects of 1alpha,25-dihydroxyvitamin D3 on the cytokine production of human peripheral blood lymphocytes. J Clin Endocrinol Metab. 1999;84:3739–44.
Correale J, Ysrraelit MC, Gaitan MI. Immunomodulatory effects of Vitamin D in multiple sclerosis. Brain. 2009;132:1146–60.
Martineau AR, Jolliffe DA, Hooper RL, Greenberg L, Aloia JF, Bergman P, et al. Vitamin D supplementation to prevent acute respiratory tract infections: systematic review and meta-analysis of individual participant data. BMJ. 2017;356:i6583.
Ginde AA, Mansbach JM, Camargo CA Jr. Association between serum 25-hydroxyvitamin D level and upper respiratory tract infection in the Third National Health and Nutrition Examination Survey. Arch Intern Med. 2009;169:384–90.
Koenig SM. Pulmonary complications of obesity. Am J Med Sci. 2001;321:249–79.
Holley HS, Milic-Emili J, Becklake MR, Bates DV. Regional distribution of pulmonary ventilation and perfusion in obesity. J Clin Invest. 1967;46:475–81.
Ray CS, Sue DY, Bray G, Hansen JE, Wasserman K. Effects of obesity on respiratory function. Am Rev Respir Dis. 1983;128:501–6.
Shailaja S, Nichelle SM, Shetty AK, Hegde BR. Comparing ease of intubation in obese and lean patients using intubation difficulty scale. Anesth Essays Res. 2014;8:168–74.
de Jongh RT, Serne EH, IJ RG, de Vries G, Stehouwer CD. Impaired microvascular function in obesity: implications for obesity-associated microangiopathy, hypertension, and insulin resistance. Circulation. 2004;109:2529–35.
Light RB. Pulmonary pathophysiology of pneumococcal pneumonia. Semin Respir Infect. 1999;14:218–26.
Cheong HS, Chang Y, Joo EJ, Cho A, Ryu S. Metabolic obesity phenotypes and risk of cellulitis: a cohort study. J Clin Med. 2019;8:953. https://doi.org/10.3390/jcm8070953.
Dupuy A, Benchikhi H, Roujeau JC, Bernard P, Vaillant L, Chosidow O, et al. Risk factors for erysipelas of the leg (cellulitis): case-control study. BMJ. 1999;318:1591–4.
Valensi P, Smagghue O, Paries J, Velayoudon P, Lormeau B, Attali JR. Impairment of skin vasoconstrictive response to sympathetic activation in obese patients: influence of rheological disorders. Metabolism. 2000;49:600–6.
Carlson JA. Lymphedema and subclinical lymphostasis (microlymphedema) facilitate cutaneous infection, inflammatory dermatoses, and neoplasia: a locus minoris resistentiae. Clin Dermatol. 2014;32:599–615.
Greene AK, Zurakowski D, Goss JA. Body mass index and lymphedema morbidity: comparison of obese versus normal-weight patients. Plast Reconstr Surg. 2020;146:402–7.
Yosipovitch G, DeVore A, Dawn A. Obesity and the skin: skin physiology and skin manifestations of obesity. J Am Acad Dermatol. 2007;56:901–16. quiz 17-20
Schramm JC, Dinh T, Veves A. Microvascular changes in the diabetic foot. Int J Low Extrem Wounds. 2006;5:149–59.
Bui UT, Finlayson K, Edwards H. Validation of predictive factors for infection in adults with chronic leg ulcers: a prospective longitudinal study. J Clin Nurs. 2020;29:1074–84.
Badawi A, Ryoo SG. Prevalence of comorbidities in the Middle East respiratory syndrome coronavirus (MERS-CoV): a systematic review and meta-analysis. Int J Infect Dis. 2016;49:129–33.
Klekotka RB, Mizgala E, Krol W. The etiology of lower respiratory tract infections in people with diabetes. Pneumonol Alergol Pol. 2015;83:401–8.
Plaeke P, De Man JG, Coenen S, Jorens PG, De Winter BY, Hubens G. Clinical- and surgery-specific risk factors for post-operative sepsis: a systematic review and meta-analysis of over 30 million patients. Surg Today. 2020;50:427–39.
Jain R, Chung SM, Jain L, Khurana M, Lau SW, Lee JE, et al. Implications of obesity for drug therapy: limitations and challenges. Clin Pharmacol Ther. 2011;90:77–89.
Roe JL, Fuentes JM, Mullins ME. Underdosing of common antibiotics for obese patients in the ED. Am J Emerg Med. 2012;30:1212–4.
Zhang LJ, Guerrero-Juarez CF, Chen SX, Zhang X, Yin M, Li F. Diet-induced obesity promotes infection by impairment of the innate antimicrobial defense function of dermal adipocyte progenitors. Sci Transl Med. 2021;13:eabb5280. https://doi.org/10.1126/scitranslmed.abb5280.
Cannon J, Rajakaruna G, Dyer J, Carapetis J, Manning L. Severe lower limb cellulitis: defining the epidemiology and risk factors for primary episodes in a population-based case-control study. Clin Microbiol Infect. 2018;24:1089–94.
Kozlowska D, Mysliwiec H, Kiluk P, Baran A, Milewska AJ, Flisiak I. Clinical and epidemiological assessment of patients hospitalized for primary and recurrent erysipelas. Przegl Epidemiol. 2016;70:575–84.
Rob F, Hercogova J. Benzathine penicillin G once-every-3-week prophylaxis for recurrent erysipelas a retrospective study of 132 patients. J Dermatolog Treat. 2018;29:39–43.
Shipman AR, Millington GW. Obesity and the skin. Br J Dermatol. 2011;165:743–50.
Metin A, Dilek N, Bilgili SG. Recurrent candidal intertrigo: challenges and solutions. Clin Cosmet Investig Dermatol. 2018;11:175–85.
Doner NYS, Ekmekci TR. Evaluation of obesity-associated dermatoses in obese and overweight individuals. Turkderm. 2011;45:146–51.
Chan MK, Chong LY, Achilles Project Working Group in Hong K. A prospective epidemiologic survey on the prevalence of foot disease in Hong Kong. J Am Podiatr Med Assoc. 2002;92:450–6.
Mirmirani P, Carpenter DM. Skin disorders associated with obesity in children and adolescents: a population-based study. Pediatr Dermatol. 2014;31:183–90.
Chu CM, Lowder JL. Diagnosis and treatment of urinary tract infections across age groups. Am J Obstet Gynecol. 2018;219:40–51.
Foxman B, Brown P. Epidemiology of urinary tract infections: transmission and risk factors, incidence, and costs. Infect Dis Clin North Am. 2003;17:227–41.
Foxman B, Gillespie B, Koopman J, Zhang L, Palin K, Tallman P, et al. Risk factors for second urinary tract infection among college women. Am J Epidemiol. 2000;151:1194–205.
Semins MJ, Shore AD, Makary MA, Weiner J, Matlaga BR. The impact of obesity on urinary tract infection risk. Urology. 2012;79:266–9.
Shill MC, Huda NH, Moain FB, Karmakar UK. Prevalence of uropathogens in diabetic patients and their corresponding resistance pattern: results of a survey conducted at diagnostic centers in dhaka, bangladesh. Oman Med J. 2010;25:282–5.
Bamgbade OA, Rutter TW, Nafiu OO, Dorje P. Postoperative complications in obese and nonobese patients. World J Surg. 2007;31:556–60. discussion 61
Saliba W, Barnett-Griness O, Rennert G. The association between obesity and urinary tract infection. Eur J Intern Med. 2013;24:127–31.
Kaspersen KA, Pedersen OB, Petersen MS, Hjalgrim H, Rostgaard K, Moller BK, et al. Obesity and risk of infection: results from the Danish Blood Donor Study. Epidemiology. 2015;26:580–9.
Nseir W, Farah R, Mahamid M, Sayed-Ahmad H, Mograbi J, Taha M, et al. Obesity and recurrent urinary tract infections in premenopausal women: a retrospective study. Int J Infect Dis. 2015;41:32–5.
Grier WR, Kratimenos P, Singh S, Guaghan JP, Koutroulis I. Obesity as a risk factor for urinary tract infection in children. Clin Pediatr (Phila). 2016;55:952–6.
Hsu PC, Chen SJ. Obesity and risk of urinary tract infection in young children presenting with fever. Medicine (Baltimore). 2018;97:e13006.
Okubo Y, Handa A. The impact of obesity on pediatric inpatients with urinary tract infections in the United States. J Pediatr Urol. 2017;13:455 e1–e5.
Zasloff M. Why are diabetics prone to kidney infections? J Clin Invest. 2018;128:5213–5.
Eichler TE, Becknell B, Easterling RS, Ingraham SE, Cohen DM, Schwaderer AL, et al. Insulin and the phosphatidylinositol 3-kinase signaling pathway regulate Ribonuclease 7 expression in the human urinary tract. Kidney Int. 2016;90:568–79.
Murtha MJ, Eichler T, Bender K, Metheny J, Li B, Schwaderer AL, et al. Insulin receptor signaling regulates renal collecting duct and intercalated cell antibacterial defenses. J Clin Invest. 2018;128:5634–46.
Kim JW, Lee YJ, Ha YS, Lee JN, Kim HT, Chun SY, et al. Secondary signs on preoperative CT as predictive factors for febrile urinary tract infection after ureteroscopic lithotripsy. BMC Urol. 2020;20:131.
Petersen A, Bressem K, Albrecht J, Thiess HM, Vahldiek J, Hamm B, et al. The role of visceral adiposity in the severity of COVID-19: Highlights from a unicenter cross-sectional pilot study in Germany. Metabolism. 2020;110:154317.
Di Bella S, Cesareo R, De Cristofaro P, Palermo A, Sanson G, Roman-Pognuz E, et al. Neck circumference as reliable predictor of mechanical ventilation support in adult inpatients with COVID-19: a multicentric prospective evaluation. Diabetes Metab Res Rev. 2021;37:e3354.
Bergmann BM, Seiden LS, Landis CA, Gilliland MA, Rechtschaffen A. Sleep deprivation in the rat: XVIII. Regional brain levels of monoamines and their metabolites. Sleep. 1994;17:583–9.
Pinheiro TA, Barcala-Jorge AS, Andrade JMO, Pinheiro TA, Ferreira ECN, Crespo TS, et al. Obesity and malnutrition similarly alter the renin-angiotensin system and inflammation in mice and human adipose. J Nutr Biochem. 2017;48:74–82.
Al Heialy S, Hachim MY, Senok A, Gaudet M, Abou Tayoun A, Hamoudi R, et al. Regulation of angiotensin-converting enzyme 2 in obesity: implications for COVID-19. Front Physiol. 2020;11:555039.
Li L, Spranger L, Soll D, Beer F, Brachs M, Spranger J, et al. Metabolic impact of weight loss induced reduction of adipose ACE-2—potential implication in COVID-19 infections? Metabolism. 2020;113:154401.
Al-Benna S. Association of high level gene expression of ACE2 in adipose tissue with mortality of COVID-19 infection in obese patients. Obes Med. 2020;19:100283.
Louie JK, Acosta M, Samuel MC, Schechter R, Vugia DJ, Harriman K, et al. A novel risk factor for a novel virus: obesity and 2009 pandemic influenza A (H1N1). Clin Infect Dis. 2011;52:301–12.
Morgan OW, Bramley A, Fowlkes A, Freedman DS, Taylor TH, Gargiullo P, et al. Morbid obesity as a risk factor for hospitalization and death due to 2009 pandemic influenza A(H1N1) disease. PLoS ONE. 2010;5:e9694.
Campitelli MA, Rosella LC, Kwong JC. The association between obesity and outpatient visits for acute respiratory infections in Ontario, Canada. Int J Obes (Lond). 2014;38:113–9.
Baik I, Curhan GC, Rimm EB, Bendich A, Willett WC, Fawzi WW. A prospective study of age and lifestyle factors in relation to community-acquired pneumonia in US men and women. Arch Intern Med. 2000;160:3082–8.
Karunanayake CP, Rennie DC, Ramsden VR, Fenton M, Kirychuk S, Lawson JA, et al. Bronchitis and its associated risk factors in first nations children. Children (Basel). 2017;4:103. https://doi.org/10.3390/children4120103.
Lee YL, Chen YC, Chen YA. Obesity and the occurrence of bronchitis in adolescents. Obesity (Silver Spring). 2013;21:E149–53.
Maccioni L, Weber S, Elgizouli M, Stoehlker AS, Geist I, Peter HH, et al. Obesity and risk of respiratory tract infections: results of an infection-diary based cohort study. BMC Public Health. 2018;18:271.
Okubo Y, Nochioka K, Testa MA. The impact of pediatric obesity on hospitalized children with lower respiratory tract infections in the United States. Clin Respir J. 2018;12:1479–84.
Pahwa P, Karunanayake CP, Rennie DC, Lawson JA, Ramsden VR, McMullin K, et al. Prevalence and associated risk factors of chronic bronchitis in First Nations people. BMC Pulm Med. 2017;17:95.
Diaz E, Rodriguez A, Martin-Loeches I, Lorente L, Del Mar Martin M, Pozo JC, et al. Impact of obesity in patients infected with 2009 influenza A(H1N1). Chest. 2011;139:382–6.
Viasus D, Pano-Pardo JR, Pachon J, Campins A, Lopez-Medrano F, Villoslada A, et al. Factors associated with severe disease in hospitalized adults with pandemic (H1N1) 2009 in Spain. Clin Microbiol Infect. 2011;17:738–46.
Coleman LA, Waring SC, Irving SA, Vandermause M, Shay DK, Belongia EA. Evaluation of obesity as an independent risk factor for medically attended laboratory-confirmed influenza. Influenza Other Respir Viruses. 2013;7:160–7.
Gottrup FMA, Hollander DA. An overview of surgical site infections: aetiology, 375 incidence and risk factors. EWMA J. 2005;5:11–5 376.
Pinkney TD, Calvert M, Bartlett DC, Gheorghe A, Redman V, Dowswell G, et al. Impact of wound edge protection devices on surgical site infection after laparotomy: multicentre randomised controlled trial (ROSSINI Trial). BMJ. 2013;347:f4305.
O’Keeffe AB, Lawrence T, Bojanic S. Oxford craniotomy infections database: a cost analysis of craniotomy infection. Br J Neurosurg. 2012;26:265–9.
Abbey M, Triantafilidis C, Topping DL. Dietary non-starch polysaccharides interact with cholesterol and fish oil in their effects on plasma lipids and hepatic lipoprotein receptor activity in rats. J Nutr. 1993;123:900–8.
Ketonen J, Pilvi T, Mervaala E. Caloric restriction reverses high-fat diet-induced endothelial dysfunction and vascular superoxide production in C57Bl/6 mice. Heart Vessels. 2010;25:254–62.
Colquitt JL, Picot J, Loveman E, Clegg AJ. Surgery for obesity. Cochrane Database Syst Rev. 2009;2:CD003641.
Savastano S, Di Somma C, Pivonello R, Tarantino G, Orio F, Nedi V, et al. Endocrine changes (beyond diabetes) after bariatric surgery in adult life. J Endocrinol Invest. 2013;36:267–79.
Kakarla VR, Nandipati K, Lalla M, Castro A, Merola S. Are laparoscopic bariatric procedures safe in superobese (BMI >/=50 kg/m2) patients? An NSQIP data analysis. Surg Obes Relat Dis. 2011;7:452–8.
Ferraz AAB, Vasconcelos CFM, Santa-Cruz F, Aquino MAR, Buenos-Aires VG, Siqueira LT. Surgical site infection in bariatric surgery: results of a care bundle. Rev Col Bras Cir. 2019;46:e2252.
Gendall KA, Raniga S, Kennedy R, Frizelle FA. The impact of obesity on outcome after major colorectal surgery. Dis Colon Rectum. 2007;50:2223–37.
Lee DW, Han SW, Cha Y, Lee KH, Kim TY, Oh DY, et al. Prognostic influence of body mass index and body weight gain during adjuvant FOLFOX chemotherapy in Korean colorectal cancer patients. BMC Cancer. 2015;15:690.
Wahl TS, Patel FC, Goss LE, Chu DI, Grams J, Morris MS. The obese colorectal surgery patient: surgical site infection and outcomes. Dis Colon Rectum. 2018;61:938–45.
Merkow RP, Bilimoria KY, McCarter MD, Bentrem DJ. Effect of body mass index on short-term outcomes after colectomy for cancer. J Am Coll Surg. 2009;208:53–61.
Healy LA, Ryan AM, Sutton E, Younger K, Mehigan B, Stephens R, et al. Impact of obesity on surgical and oncological outcomes in the management of colorectal cancer. Int J Colorectal Dis. 2010;25:1293–9.
Okabayashi T, Nishimori I, Yamashita K, Sugimoto T, Yatabe T, Maeda H, et al. Risk factors and predictors for surgical site infection after hepatic resection. J Hosp Infect. 2009;73:47–53.
Gervaz P, Bandiera-Clerc C, Buchs NC, Eisenring MC, Troillet N, Perneger T, et al. Scoring system to predict the risk of surgical-site infection after colorectal resection. Br J Surg. 2012;99:589–95.
Greenblatt DY, Kelly KJ, Rajamanickam V, Wan Y, Hanson T, Rettammel R, et al. Preoperative factors predict perioperative morbidity and mortality after pancreaticoduodenectomy. Ann Surg Oncol. 2011;18:2126–35.
Su Z, Koga R, Saiura A, Natori T, Yamaguchi T, Yamamoto J. Factors influencing infectious complications after pancreatoduodenectomy. J Hepatobiliary Pancreat Sci. 2010;17:174–9.
Figuerola-Tejerina A, Rodriguez-Caravaca G, Bustamante-Munguira J, Maria San Roman-Montero J, Duran-Poveda M. Epidemiological surveillance of surgical site infection and its risk factors in cardiac surgery: a prospective cohort study. Rev Esp Cardiol (Engl Ed). 2016;69:842–8.
Dodds Ashley ES, Carroll DN, Engemann JJ, Harris AD, Fowler VG Jr, Sexton DJ, et al. Risk factors for postoperative mediastinitis due to methicillin-resistant Staphylococcus aureus. Clin Infect Dis. 2004;38:1555–60.
Lu K, Zhang J, Cheng J, Liu H, Yang C, Yin L, et al. Incidence and risk factors for surgical site infection after open reduction and internal fixation of intra-articular fractures of distal femur: a multicentre study. Int Wound J. 2019;16:473–8.
Dias M, Dick A, Reynolds RM, Lahti-Pulkkinen M, Denison FC. Predictors of surgical site skin infection and clinical outcome at caesarean section in the very severely obese: a retrospective cohort study. PLoS ONE. 2019;14:e0216157.
Acknowledgements
The scientific assistance of Panta Rei Impresa Sociale srl (https://www.panta-rei.eu/pantarei/) is gratefully appreciated.
Funding
The study was funded by the Ministry of University and Research Grants PRIN 2020NCKXBR (to AC), entitled ‘Suscettibilità alle malattie infettive nell’obesità: una valutazione endocrina, traslazionale e sociologica (SIDERALE)’.
Author information
Authors and Affiliations
Contributions
Conceptualization: GP; Data curation: GP and AL; Writing—original draft preparation: GP and AL; Writing—review and editing: CG, GM, LB, AC; Supervision: AC; funding acquisition: CG and AC. All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Pugliese, G., Liccardi, A., Graziadio, C. et al. Obesity and infectious diseases: pathophysiology and epidemiology of a double pandemic condition. Int J Obes 46, 449–465 (2022). https://doi.org/10.1038/s41366-021-01035-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41366-021-01035-6
This article is cited by
-
Very low-calorie ketogenic diet (VLCKD) in the management of hidradenitis suppurativa (Acne Inversa): an effective and safe tool for improvement of the clinical severity of disease. Results of a pilot study
Journal of Translational Medicine (2024)
-
Economic costs of obesity: a systematic review
International Journal of Obesity (2024)
-
The demographic, laboratory and genetic factors associated with long Covid-19 syndrome: a case–control study
Clinical and Experimental Medicine (2024)
-
Obesity as a clinical predictor for severe manifestation of dengue: a systematic review and meta-analysis
BMC Infectious Diseases (2023)
-
A review of the role of liposome-encapsulated phytochemicals targeting PPAR Ɣ and associated pathways to combat obesity
3 Biotech (2023)