Abstract
Background
Obesity is among the main determinants of nonalcoholic fatty liver disease progression towards severe liver disease (SLD). However, risk factors for SLD in individuals with obesity have not been examined.
Objectives
To identify the independent risk factors for SLD among participants with obesity from the UK Biobank.
Methods
A total of 80,224 UK Biobank participants with obesity (body mass index[BMI] > 30 kg/m2) and 242,822 without obesity, of European descent without clinical history of liver disease and liver cancer were prospectively followed for the onset of SLD, defined as a composite diagnosis of cirrhosis, decompensated liver disease, hepatocellular carcinoma and/or liver transplantation. Risk factors for incident SLD were assessed by Cox proportional hazards models. Different clinical phenotypes were derived by latent class analysis (LCA).
Results
Obesity conferred a 2.6-fold increased risk for SLD that was abolished after the inclusion of waist circumference (WC) in the model. Among individuals with obesity, age (adjusted hazard ratio [aHR] 1.05, 95%CI 1.03–1.07, p = 3.9 * 10−7), type 2 diabetes (aHR 2.18, 95%CI 1.55–3.05, p = 6.2 * 10−6), PNPLA3 rs738409 (aHR 1.59, 95%CI 1.33–1.9, p = 3.1 * 10−7) and WC (aHR 1.04, 95%CI 1.02–1.06, p = 8.5 * 10−6) were independent predictors of SLD. BMI category-specific WC thresholds allowed a better risk stratification compared to traditional ones. By LCA, the clinical phenotype at highest risk for SLD was that with BMI < 35 kg/m2 and WC above BMI-category specific thresholds.
Conclusions
Age, WC, type 2 diabetes, and the PNPLA3 variant are the main risk factors for SLD in individuals with obesity. WC is the principal mediator of SLD risk conveyed by increased BMI. BMI category-specific WC-thresholds may refine the SLD risk more accurately than traditional thresholds.
Similar content being viewed by others
Introduction
Nonalcoholic fatty liver disease (NAFLD) is emerging as the main reason for referrals to hepatology services and as the first cause of cirrhosis and hepatocellular carcinoma in Western countries [1, 2]. NAFLD is often associated with metabolic disorders, including obesity and type 2 diabetes among the most important [3]. Indeed, the global prevalence of NAFLD is estimated 25–30% worldwide but it ranges between ∼70% and ∼80% in individuals with obesity [4,5,6], rising up to 90% in those with morbid obesity [6]. Moreover, obesity is also a risk factor for more severe histological lesions, including higher NAFLD activity score and fibrosis [7, 8], and for progression to cirrhosis [7] and development of hepatocellular carcinoma (HCC) [9, 10].
Indeed, the latest EASL/EASD/EASO guidelines recommend screening for liver steatosis as a part of the standard evaluation of subjects with obesity and, in those with steatosis, also the evaluation of noninvasive markers of fibrosis [2]. However, ~500 million individuals are estimated to be obese [11], making this proposal unfeasible. ALT values do not correlate with histological findings and are unhelpful in both the diagnosis of NAFLD and determining disease severity [12]. The identification of risk factors to stratify individuals with obesity that will progress to severe liver disease (SLD), i.e., cirrhosis, cirrhosis decompensation, HCC, and liver-related death, emerges as crucial. Indeed, it may allow to prioritize subjects with obesity to the screening for NAFLD and advanced liver fibrosis. However, to the best of our knowledge, the identification of risk factors for SLD specifically in individuals with obesity from the general population has not been done.
Although many individuals with obesity will develop metabolic diseases, a sizeable part of them will not, a phenomenon that has been described as metabolically healthy obesity [13]. High body mass index (BMI) levels associate with an increased risk of cardiometabolic disease [14]. Abdominal obesity, as measured by waist circumference (WC), enables further refinement of this risk [15]. Moreover, the association of WC with cardiovascular disease varies across the BMI categories [15], leading to a recent proposal to consider BMI category-specific WC thresholds [16]. Notably, these BMI category-specific thresholds have never been verified with respect to the risk of chronic liver disease.
Therefore, in the present study, we aimed to identify risk factors for SLD in a large, population-based cohort of individuals with obesity. A further aim was to highlight the most relevant phenotypic profiles of subjects with obesity in terms of co-occurrence of the main SLD risk factors emerging from the analysis, and to verify their different risk of developing SLD.
Methods
Study population and data collection
We used data from the UK Biobank, a large prospective cohort including over 500,000 participants (age 40–69 years) recruited between 2006 and 2010 from 22 assessment centers throughout the UK. Study design and methods of the UK Biobank have been described in detail previously [17]. Potential participants were identified from the National Health Service patient registers. At the baseline assessment visit, they completed a touch-screen self-administered questionnaire and a computer-assisted interview regarding medical history, current pharmacological therapy, sociodemographic characteristics, smoking status, alcohol consumption, dietary habits, physical activity, and family history of major diseases. Baseline anthropometric measures (e.g., height, weight, and waist circumference) were assessed by trained staff using standardized procedures. Blood samples were collected for genome-wide genotyping and biochemical analyses, including serum glucose, total cholesterol, alanine aminotransferase (ALT), aspartate aminotransferase (AST), gamma-glutamyltransferase (GGT), and albumin (AU5800, Beckman Coulter). The protocol for sample collecting, processing, and storage was developed using a highly automated and validated approach [18]. Further information about the study protocol and methods is available on the UK Biobank website (https://www.ukbiobank.ac.uk/). The UK Biobank study has been approved by the North West Multicenter Research Ethics Committee (reference number 11/NW/0382). All participants provided written informed consent to the study.
Sample selection
From the total study population (N 502,536), we excluded subjects with: 1) self-reported history of liver disease (N 3989), alcohol abuse (N 682) or excessive alcohol consumption (≥30 g/die and ≥20 g/die for men and women, respectively—N 87,935); 2) hospital diagnosis of liver disease occurred before the baseline visit and defined according to the International Classification of Diseases 10th edition (ICD-10) (N 1,616); 3) diagnosis of any cancer (except for precancerous conditions of the cervix) both self-reported or based on cancer registry and occurred before the baseline assessment visit (N 51,822). Thereafter, we removed participants with non-European descent (N 24,846) and those with withdrawn consent (N 30). Finally, subjects with missing BMI data were excluded (N 1570). A total of 330,046 participants were included for the final analyses [with obesity (BMI > 30 kg/m2) 80,224 (24.3%), without obesity (BMI≤30 kg/m2) 242,822 (75.7%)]. Details of baseline exclusion criteria have been provided in Supplementary Tables 1 and 2.
Baseline covariates and comorbidities
Height and weight were measured using the Seca 202 height measure (Seca, Hamburg, Germany) and the Tanita BC-418 MA body composition analyzer (Tanita Europe, Amsterdam, Netherlands), respectively. Body mass index (BMI) was calculated by dividing the weight (kg) by the square of the height (m2). Waist circumference was measured at the umbilicus level using the Wessex non-stretchable sprung tape measure (Wessex, UK). Smoking status was categorized into two groups: current smoking and never/former smoking. Baseline type 2 diabetes was defined by at least one of the following criteria: 1) self-reported history of type 2 or unspecified diabetes; 2) hospital diagnosis of type 2 or unspecified diabetes occurred before the baseline assessment visit (ICD-10 E11, E14); 3) current insulin treatment and/or use of oral hypoglycemic drugs; 4) serum glucose level ≥11.1 mmol/L (200 mg/dL); 5) HbA1c ≥ 48 mmol/mol (6.5%). Baseline dyslipidemia was defined as self-reported history of abnormal values of total, low or high-density lipoproteins (LDL, HDL) cholesterol or triglycerides, or use of lipid-lowering drugs. Similarly, baseline hypertension was defined as self-reported history of hypertension or use of anti-hypertensive drugs. Baseline cardiovascular disease was defined as self-reported history of angina, myocardial infarction, stroke, or transient ischemic attack. Detailed information about genotyping and arrays used in the UK Biobank study has been provided elsewhere [19]. The patatin-like phospholipase domain-containing 3 (PNPLA3) rs738409 C>G (p.I148M), transmembrane 6 superfamily member 2 (TM6SF2) rs58542926 C>T (p.E167K), membrane-bound O-acyltransferase domain-containing 7 (MBOAT7) rs641738 C>T, glucokinase regulator (GCKR) rs1260326 C>T (p.P446L) and hydroxysteroid 17-beta dehydrogenase 13 (HSD17B13) rs72613567:TA were genotyped using two very similar arrays (i.e., Affymetrix UK BiLEVE and UK Biobank Axiom arrays) and coded as 0, 1, or 2 for non-carriers, heterozygous carriers, and homozygous carriers of the minor allele, respectively.
Outcome ascertainment
Follow-up data on health-related events and mortality were obtained through linkage of the National Health Service records, including in-hospital admissions, death register, and cancer register. The outcome of interest was incident SLD, defined as a composite diagnosis of cirrhosis, decompensated liver disease (i.e., esophageal varices with or without bleeding, portal hypertension, hepatorenal syndrome, liver failure), hepatocellular carcinoma, and/or liver transplantation (ICD-10 C22.0, I85.0, I85.9, K70.3, K70.4, K72.1, K72.9, K74.1, K74.2, K74.6, K76.6, K76.7, Z94.4) in any of the aforementioned records. A list of all the diagnoses used to define SLD is presented in Supplementary Table 3. The follow-up began at the date of baseline assessment visit and ended at the date of SLD, death, competing non-NAFLD diagnoses, or last update of the registries (31 January 2018), whichever occurred first. Competing non-NAFLD diagnoses were considered as the occurrence of hospital diagnosis of chronic viral hepatitis, Wilson disease, hemochromatosis, drug-induced liver injury, autoimmune hepatitis, inflammatory liver diseases, and/or chronic biliary disorders (ICD-10 B18, B19, E83.0, E83.1, K71, K74.3, K74.4, K74.5, K75.2, K75.3, K75.4, K75.8, K75.9) during the follow-up.
Statistical analysis
The general characteristics of the study population were presented by means of descriptive statistics. Cox proportional hazard regression models were fitted to investigate the impact of obesity on the occurrence of SLD in the overall population, and of other factors associated with SLD specifically in the subset of subjects with obesity. The strength of associations was expressed by means of hazard ratio (HR) with 95% confidence intervals (CI). Multivariable models were carried out to correct for potential confounders, and included variables associated with outcome with a p value < 0.1 at univariate analysis. The proportional hazard assumption was verified through the inspection of the Schoenfeld residuals. Risk estimates of different WC thresholds for the development of SLD were also computed applying Cox proportional regression models corrected for age and gender. Finally, we assessed whether the main risk factors for SLD aggregated into distinct classes (clinical phenotypes) using the latent class analysis (LCA). We used this method to test the hypothesis that the UKBB population of subjects with obesity comprises N sub-populations (classes) characterized by the co-occurrence of risk factors. N was fixed at 3 for both genders, corresponding to the value that maximized the goodness-of-fit (evaluated by Bayesian information factor -BIC-) of models with different class numbers (Supplementary Fig. 1). Once the classes were obtained, the specific incidence of SLD within each clinical phenotype was computed. All cumulative incidence curves for SLD occurrence were calculated using Aalen-Johansen estimator, with incident non-NAFLD liver disease and mortality entered as competing event for SLD. Analyses were stratified according to gender and conducted with R statistics 4.0.2 (R Foundation for Statistical Computing, Vienna, Austria).
Results
General characteristics of the study population and risk of SLD in subjects with Vs without obesity
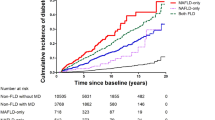
After exclusions, a total of 80,224 subjects with obesity and 242,822 without obesity were analyzed. At baseline, the mean age of individuals with obesity was 59.6 years with a mean BMI of 34.1 kg/m2, 55% were female and 12% had type 2 diabetes (Table 1). Compared to subjects without obesity, subjects with obesity had a higher waist circumference (WC), and an increased prevalence of type 2 diabetes, hypertension, and dyslipidemia (Supplementary Table 4). During a median follow-up of 9.0 years (IQR 8.3-9.7), 318 subjects with obesity (0.40%) and 379 without obesity (0.15%) developed SLD (p = 1.9 * 10−33). Individuals with obesity showed a higher cumulative SLD incidence followed by overweight, as compared to normal-weight individuals (Fig. 1). Similar results were observed in the cohorts stratified by gender (Fig. 1).
Obesity conferred a greater than 2.5 fold increase in the risk for SLD that was mitigated by adjusting for age, gender, type 2 diabetes, hypertension, and dyslipidemia (Table 2, adjusted model 1). However, the further inclusion of WC in the model abolished this association (Table 2, adjusted model 2). Notably, this result was consistent also when only WC was added in the model (Table 2, adjusted model 3). These results suggest that the visceral adiposity mediates the risk of SLD conferred by BMI.
Risk factors for SLD in individuals with obesity
Subjects with obesity developing SLD during follow-up were older, more frequently men and smokers, with higher BMI, WC, and prevalence of type 2 diabetes, hypertension, dyslipidemia, and cardiovascular disease (Table 1). Concerning biochemical parameters, they had higher liver enzymes and bilirubin levels, while showed lower total, LDL, and HDL cholesterol, platelet, and albumin levels, which are known proxies of liver function. Among genetic factors, this group was enriched in carriers of the PNPLA3 rs738409 and TM6SF2 rs58542926 variants.
Univariate and multivariate Cox regression analyses of risk factors for incident SLD in subjects with obesity are presented in Table 3 and in Supplementary Table 5. Age, WC, type 2 diabetes, hypertension, smoking, and the PNPLA3 and TM6SF2 variants were independently associated with SLD in the overall population. In the analysis stratified by gender, age, WC, type 2 diabetes and the PNPLA3 variant were confirmed independent predictors of SLD in both genders. Conversely, the associations of hypertension, smoking, and the TM6SF2 variant were not present likely due to a lower statistical power after stratification for gender.
Abdominal adiposity as a main determinant of the risk of SLD
Since WC, and not BMI, was independently associated with SLD also when restricting the analysis to the individuals with obesity, we aimed to verify if the BMI category-specific WC thresholds were able to improve risk stratification for SLD. In Fig. 2 and in Supplementary Fig. 2, the cumulative incidences of SLD at the end of follow-up stratified by categories of BMI (30–34.9 Vs ≥ 35 kg/m2), and to the different thresholds of WC, the traditional (≥88 cm for women and ≥102 cm for men), and those BMI category-specific (BMI 30–34.9 kg/m2: ≥105 cm for women and ≥110 cm for men; BMI ≥ 35 kg/m2: ≥115 cm for women and ≥125 cm for men) [15, 16], are presented. In the overall population, for each category of BMI, there was a higher cumulative incidence of SLD when considering higher WC thresholds, while, when considering WC thresholds, a higher BMI category was associated with slightly increased SLD incidence only when considering the lowest WC threshold, while there was a trend of the higher BMI category to be even protective when considering the highest WC thresholds.
Numbers at the base of bars represent the total population in each group along with percentage of subjects over the WC threshold. *p < 0.05, **p < 0.01 for comparison of incidence of severe liver disease across BMI groups. P value of 6 * 10−7 and 6 * 10−3, for comparison of incidence of severe liver disease across different thresholds of waist circumference in BMI 30–34.9 and ≥35 kg/m2, respectively. All comparisons were carried out by means of χ2 test.
When the analysis was stratified by gender, for any given BMI class, the increase in the incidence of SLD according to WC thresholds was more evident in women than in men, in whom different SLD incidences according to WC thresholds were observed only in subjects with BMI 30–34.9 and not in those with BMI ≥ 35 kg/m2. The trend towards a protective effect against SLD of BMI ≥ 35 kg/m2 in those with the highest WC thresholds was more evident in men than in women.
Considering these findings in the obese group, we analyzed HRs for SLD of subjects with obesity as compared to those without obesity stratified by WC thresholds, overall and according to gender (Table 4). Consistent with the findings in Fig. 2, the BMI category-specific thresholds were able to refine the risk stratification for SLD, more accurately in BMI 30–34.9 kg/m2 than in BMI ≥ 35 kg/m2, and more accurately in women than in men. Moreover, for subjects with obesity with WC above the highest thresholds, having a BMI ≥ 35 kg/m2 obesity was again protective as compared to BMI 30–34.9 kg/m2.
Clinical phenotypes of subjects with obesity
By means of LCA including gender-specific risk factors for SLD at univariate analysis (age, BMI, WC, hypertension, PNPLA3 and type 2 diabetes in both genders, plus TM6SF2 only in men and smoke only in women), three clinical phenotypes were identified in men and in women (Supplementary Table 6). The first (LC1) and the third (LC3) ones showed similar characteristics in the two genders. Hence, LC1 included 21,477 (59.6%) men and 25,481 (57.6%) women mostly characterized by BMI < 35 kg/m2 (95% of men and 100% of women) and WC below BMI category-specific thresholds (83% of men and 100% of women), with the lowest prevalence of cardiometabolic comorbidities. Conversely, LC3 was made up of 7,129 (19.8%) men and 3,562 (8.1%) women with BMI < 35 kg/m2 (100% of men and women) and mostly with WC above BMI category-specific thresholds (74% of men and 99% of women). Subjects in LC3 were also older (age ≥ 60 in 82% of men and 52% of women) and with the highest prevalence cardiometabolic comorbidities. The characteristics of the last clinical phenotype (LC2) differed across genders. Indeed, in men, LC2 included 7,412 (20.6%) subjects with BMI ≥ 35 kg/m2 (100%) and WC above BMI category-specific thresholds (87%); conversely, in women, it included 15,163 (34.3%) subjects with BMI ≥ 35 kg/m2 (100%) and WC below BMI category-specific thresholds (93%). In both genders, the prevalence of cardiometabolic comorbidities in LC2 was significantly higher than in LC1 and, in men, it was also lower than in LC3. Supplementary Fig. 3 (left panel) visually represents the characteristics of the aforementioned phenotypes according to the main risk factors for SLD.
Overall, subjects in LC3 showed the highest cumulative incidence of SLD at the end of follow-up in both genders (Supplementary Fig. 3, central and right panels), followed by those in LC2 and LC1 (men: 2.0% Vs 0.8% Vs 0% p = 2 * 10−16; women:1.5% Vs 0.4 Vs 0% p = 2 * 10−14) (Supplementary Fig. 3 and Supplementary Table 6).
Discussion
In the present study, we examined for the first time risk factors for SLD in individuals with obesity. We found that age, WC, type 2 diabetes and the PNPLA3 variant are independent determinants of adverse hepatic outcomes in both genders. Importantly, abdominal adiposity, measured by WC, emerged as the main mediator of SLD risk associated with BMI. Finally, by latent class analysis, the clinical phenotype with the highest SLD risk included individuals with obesity with BMI < 35 kg/m2 and WC above BMI category-specific thresholds.
Obesity and type 2 diabetes are the main risk factors for NAFLD and they are also associated with a more aggressive liver disease, i.e., the development of nonalcoholic steatohepatitis and fibrosis progression [20]. Accordingly, they have been clearly recognized among the diseases in which NAFLD should be systematically investigated, and, if present, further assessed for its severity in terms of fibrosis [2]. However, given the epidemic of obesity and type 2 diabetes, guidelines are likely to fall on deaf ears if their message is not further refined by risk factors to prioritize individuals for the screening. In this regard, previous studies have identifed risk factors for SLD in diabetic participants of large population based studies from Sweden and UK [21, 22]. However, to date, there is no study specifically aimed to verify the determinants of SLD in individuals with obesity.
In the present work, we showed that the incidence of SLD is higher in overweight and in individuals with obesity. Obesity was a significant risk factor for SLD even after adjusting for confounders, including age, gender, type 2 diabetes, hypertension and dyslipidemia. However, the association between obesity and SLD was abolished when WC was included as a covariate in the analysis, and, most notably, even when the model was adjusted only for WC. These results suggest that abdominal adiposity is the main determinant of SLD risk conveyed by obesity. Consistent with this notion, WC is robustly associated with all-cause and cardiovascular mortality [23,24,25,26]. With respect to liver disease, literature on the association between WC and SLD is scarce and somehow controversial [27], but a recent metanalysis suggests that measures of central obesity are better than BMI at prognosticating SLD [27].
BMI was associated with SLD also when the analysis was restricted to subjects with obesity. However, in the multivariate models, the association with BMI was again abolished, whereas age, WC, type 2 diabetes, hypertension, smoking, and the PNPLA3 and TM6SF2 variants remained independently associated with SLD. Moreover, after gender stratification, only age, WC, type 2 diabetes and the PNPLA3 variant were independent predictors of SLD in both genders.
Among NAFLD comorbidities, type 2 diabetes seems to be the strongest risk factor for progression of liver disease to its long-term complications, i.e., cirrhosis and hepatocellular carcinoma, and for mortality [28]. In this context, we demonstrate that type 2 diabetes is still the most dangerous comorbidity with respect to the risk of adverse hepatic outcomes even when the analysis is limited to subjects with obesity. Concerning genetic variants, NAFLD is a multifactorial disease whose heritability estimates has been found to range from 20% to 70%, depending on different type and design of the studies [29,30,31]. Moreover, as we reviewed elsewhere [32], most of the variants which have been more consistently associated with liver fat content, i.e., PNPLA3 rs738409, TM6SF2 rs58542926, and MBOAT7 rs641738, have been subsequently associated also with development of NASH and fibrosis, suggesting that the amount of hepatic fat is a crucial driver of disease progression [33, 34]. Accordingly, a polygenic risk score designed for its capability to predict hepatic fat content has been associated with liver injury, inflammation and fibrosis [33]. Very recently, we have found that this same score was also able to predict the development of HCC [35] and to improve the risk stratification and prediction of SLD combined with non-invasive clinical fibrosis scores [36]. The PNPLA3 polymorphism, which is recognized as the strongest determinant of interindividual and ethnicity-related differences in hepatic fat content, is here the main genetic determinant of adverse hepatic outcomes in subjects with obesity. In both genders, each PNPLA3 variant allele was associated with about 50% increased risk of SLD. Concerning the other polymorphisms, the association of TM6SF2 variant was abolished after stratification by gender probably due to a lack of power.
In our study, abdominal adiposity, i.e., WC, was an independent driver of disease progression in individuals with obesity. The gender‐specific thresholds were originally developed in a large sample of white men and women in which a WC of 102 cm and 88 cm, respectively, corresponded to a BMI of 30 kg/m2 [37]. Thus, these thresholds were designed to be used in place of BMI as an alternative way to identify those in need of weight management rather than for their relationship with health risk. Accordingly, in this study, ~84% and ~99% of obese with BMI 30–34 kg/m2 and ≥35 kg/m2, respectively, were above these thresholds (see Fig. 2). Although redefining WC thresholds based on the underlying class of obesity improves the risk stratification and prediction for cardiovascular events [15, 16], these BMI category-specific WC thresholds have never been investigated before with respect to the occurrence of SLD. Here we found that BMI category-specific thresholds allow a refinement in risk stratification for SLD, which is higher in women and in those with BMI 30–34 kg/m2. Notably, for subjects with the highest WC thresholds, a BMI ≥ 35 kg/m2 was even protective with respect to BMI 30-34 kg/m2. Results obtained by latent class analysis recapitulated these findings. In both genders, the obese clinical phenotype showing the highest rates of cardiometabolic comorbidities and the highest risk for SLD was that with BMI < 35 kg/m2 and WC above BMI category specific thresholds. Indeed, for any given WC, the higher the BMI the lower the risk of death [38], likely because larger BMI may be driven by an increased accumulation of subcutaneous adipose tissue in the lower body that is known to be metabolically healthy [14]. This is consistent with the finding that, while in univariate analysis BMI shows a strong positive association with visceral adipose tissue, after adjustment for WC the association becomes even inverse and BMI is positively associated with lower body subcutaneous adipose tissue and skeletal muscle mass [39]. This observation is consistent with the negative association commonly observed between BMI and morbidity and mortality after adjustment for WC.
This study has several strengths: a) the large sample size, with inclusion of more than 80,000 subjects with obesity and more than 240,000 without obesity from the general population; b) the prospective design; c) the extensive analysis of genetic and acquired risk factors in all participants; d) the use of standardized procedures and of a centrally validated protocol for blood samples collection, processing and storage in the UK Biobank. The present study has also some limitations. First, asymptomatic advanced liver disease (e.g., compensated cirrhosis or new-onset HCC on NAFLD) may have been overlooked. However, liver-related diagnoses were linked from multiple registers (i.e., hospital records, death register and cancer register) to limit this bias. Similarly, even in a minority of cases, subjects identified with portal hypertension or varices may be affected by non-cirrhotic rather than cirrhotic portal hypertension. We have also to aknowledge that the WC thredsholds we have used were originally derived for the prediction of cardiovascular disease. However, it was not within the aims of our study, nor consistent with its design, to derive and validate novel thresholds for the prediction of SLD in subjects with obesity. Rather, we aimed to provide proof of concept that BMI-category specific WC thresholds may refine risk stratification for SLD in subjects with obesity. Finally, the vast majority of the UK Biobank individuals are of European descent, so future studies are needed for extending these findings to different ethnic groups.
In conclusion, the present is the first study to analyze the inborn and acquired risk factors for SLD in individuals with obesity from the UK Biobank. Age, WC, type 2 diabetes and the PNPLA3 variant are the strongest predictors of SLD in these indivduals. Further studies are needed to define customized screening and/or intervention approaches for SLD in subjects with obesity.
Data sharing statement
UK Biobank data are available through a procedure described at http://www.ukbiobank.ac.uk/using-the-resource/.
References
Younossi Z, Anstee QM, Marietti M, Hardy T, Henry L, Eslam M, et al. Global burden of NAFLD and NASH: trends, predictions, risk factors and prevention. Nat Rev Gastroenterol Hepatol. 2018;15:11–20.
European Association for the Study of Diabetes (EASD), European Association for the Study of Obesity (EASO). EASL-EASD-EASO Clinical Practice Guidelines for the management of non-alcoholic fatty liver disease. J Hepatol. 2016;64:1388–402.
Bellentani S, Saccoccio G, Masutti F, Crocè LS, Brandi G, Sasso F, et al. Prevalence of and risk factors for hepatic steatosis in Northern Italy. Ann Intern Med. 2000;132:112–7.
Berg EHvanden, Amini M, Schreuder TCMA, Dullaart RPF, Faber KN, Alizadeh, et al. Prevalence and determinants of non-alcoholic fatty liver disease in lifelines: a large Dutch population cohort. PLOS ONE. 2017;12:e0171502.
Williams CD, Stengel J, Asike MI, Torres DM, Shaw J, Contreras M, et al. Prevalence of nonalcoholic fatty liver disease and nonalcoholic steatohepatitis among a largely middle-aged population utilizing ultrasound and liver biopsy: a prospective study. Gastroenterology. 2011;140:124–31.
Fazel Y, Koenig AB, Sayiner M, Goodman ZD, Younossi ZM. Epidemiology and natural history of non-alcoholic fatty liver disease. Metabolism. 2016;65:1017–25.
Powell EE, Cooksley WG, Hanson R, Searle J, Halliday JW, Powell LW. The natural history of nonalcoholic steatohepatitis: a follow-up study of forty-two patients for up to 21 years. Hepatology. 1990;11:74–80.
Sookoian S, Pirola CJ. Systematic review with meta-analysis: the significance of histological disease severity in lean patients with nonalcoholic fatty liver disease. Aliment Pharmacol Ther. 2018;47:16–25.
Nair S, Mason A, Eason J, Loss G, Perrillo RP. Is obesity an independent risk factor for hepatocellular carcinoma in cirrhosis? Hepatology. 2002;36:150–5.
Finucane MM, Stevens GA, Cowan M, Danaei G, Lin JK, Paciorek CJ, et al. National, regional, and global trends in body mass index since 1980: Systematic analysis of health examination surveys and epidemiological studies with 960 country-years and 9.1 million participants. Lancet. 2011;377:557–67.
Iacobini C, Pugliese G, Blasetti Fantauzzi C, Federici M, Menini S. Metabolically healthy versus metabolically unhealthy obesity. Metabolism. 2019;92:51–60.
Mofrad P, Contos MJ, Haque M, Sargeant C, Fisher RA, Luketic VA, et al. Clinical and histologic spectrum of nonalcoholic fatty liver disease associated with normal ALT values. Hepatology. 2003;37:1286–92.
Jacobs EJ, Newton CC, Wang Y, Patel AV, McCullough ML, Campbell PT, et al. Waist circumference and all-cause mortality in a large US cohort. Arch Intern Med. 2010;170:1293–301.
Khan SS, Ning H, Wilkins JT, Allen N, Carnethon M, Berry JD, et al. Association of body mass index with lifetime risk of cardiovascular disease and compression of morbidity. JAMA Cardiol. 2018;3:280–7.
Ardern CI, Janssen I, Ross R, Katzmarzyk PT. Development of health-related waist circumference thresholds within BMI categories. Obes Res. 2004;12:1094–103.
Ross R, Neeland IJ, Yamashita S, Shai I, Seidell J, Magni P, et al. Waist circumference as a vital sign in clinical practice: a Consensus Statement from the IAS and ICCR Working Group on Visceral Obesity. Nat Rev Endocrinol. 2020;16:177–89.
Sudlow C, Gallacher J, Allen N, Beral V, Burton P, Danesh J, et al. UK biobank: an open access resource for identifying the causes of a wide range of complex diseases of middle and old age. PLoS Med. 2015 Mar;12:e1001779.
Elliott P, Peakman TC. UK Biobank. The UK Biobank sample handling and storage protocol for the collection, processing and archiving of human blood and urine. Int J Epidemiol. 2008;37:234–44.
Bycroft C, Freeman C, Petkova D, Band G, Elliott LT, Sharp K, et al. The UK Biobank resource with deep phenotyping and genomic data. Nature. 2018;562:203–9.
Huang DQ, El-Serag HB, Loomba R. Global epidemiology of NAFLD-related HCC: trends, predictions, risk factors and prevention. Nat Rev Gastroenterol Hepatol. 2021;18:223–238. https://doi.org/10.1038/s41575-020-00381-6.
Björkström K, Franzén S, Eliasson B, Miftaraj M, Gudbjörnsdottir S, Trolle-Lagerros Y, et al. Risk factors for severe liver disease in patients with type 2 diabetes. Clin Gastroenterol Hepatol. 2019;17:2769–2775.e4.
Tavaglione F, De Vincentis A, Jamialahmadi O, Pujia R, Spagnuolo R, Picardi A, et al. Inborn and acquired risk factors for severe liver disease in Europeans with type 2 diabetes from the UK Biobank. JHEP Rep. 2021;100262.
Pischon T, Boeing H, Hoffmann K, Bergmann M, Schulze MB, Overvad K, et al. General and abdominal adiposity and risk of death in Europe. N Engl J Med. 2008;359:2105–20.
Cerhan JR, Moore SC, Jacobs EJ, Kitahara CM, Rosenberg PS, Adami H-O, et al. A pooled analysis of waist circumference and mortality in 650,000 adults. Mayo Clin Proc. 2014;89:335–45.
Zhang C, Rexrode KM, van Dam RM, Li TY, Hu FB. Abdominal obesity and the risk of all-cause, cardiovascular, and cancer mortality: sixteen years of follow-up in US women. Circulation. 2008;117:1658–67.
Song X, Jousilahti P, Stehouwer CDA, Söderberg S, Onat A, Laatikainen T, et al. Comparison of various surrogate obesity indicators as predictors of cardiovascular mortality in four European populations. Eur J Clin Nutr. 2013;67:1298–302.
Jarvis H, Craig D, Barker R, Spiers G, Stow D, Anstee QM, et al. Metabolic risk factors and incident advanced liver disease in non-alcoholic fatty liver disease (NAFLD): a systematic review and meta-analysis of population-based observational studies. PLoS Med [Internet]. 2020.;17(4). https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7192386/. Accessed 22 Mar 2021.
Younossi ZM. Non-alcoholic fatty liver disease—a global public health perspective. J Hepatol. 2019;70:531–44.
Sookoian S, Pirola CJ. Genetic predisposition in nonalcoholic fatty liver disease. Clin Mol Hepatol. 2017;23:1–12.
Jamialahmadi O, Mancina RM, Ciociola E, Tavaglione F, Luukkonen PK, Baselli G. et al.Exome-wide association study on alanine aminotransferase identifies sequence variants in the GPAM and APOE associated with fatty liver disease.Gastroenterology. 2020;160:1634–1646.e7. https://doi.org/10.1053/j.gastro.2020.12.023. Epub 2021 Feb 6.
De Vincentis A, Mancina RM, Pihlajamäki J, Männistö V, Petta S, Dongiovanni P, et al. Genetic variants in the MTHFR are not associated with fatty liver disease. Liver Int.2020;40:1934–40.
Vespasiani-Gentilucci U, Gallo P, Dell’Unto C, Volpentesta M, Antonelli-Incalzi R, Picardi A. Promoting genetics in non-alcoholic fatty liver disease: combined risk score through polymorphisms and clinical variables. World J Gastroenterol. 2018;24:4835–45.
Dongiovanni P, Stender S, Pietrelli A, Mancina RM, Cespiati A, Petta S, et al. Causal relationship of hepatic fat with liver damage and insulin resistance in nonalcoholic fatty liver. J Intern Med. 2018;283:356–70.
Romeo S, Sanyal A, Valenti L. Leveraging human genetics to identify potential new treatments for fatty liver disease. Cell Metab. 2020;31:35–45.
Bianco C, Jamialahmadi O, Pelusi S, Baselli G, Dongiovanni P, Zanoni I, et al. Non-invasive stratification of hepatocellular carcinoma risk in non-alcoholic fatty liver using polygenic risk scores. J Hepatol. 2021;74:775–82.
De Vincentis A, Tavaglione F, Jamialahmadi O, Picardi A, Antonelli Incalzi R, Valenti L, et al. A polygenic risk score to refine risk stratification and prediction for severe liver disease by clinical fibrosis scores. Clin Gastroenterol Hepatol. 2021;S1542-3565:00595–4.
Lean ME, Han TS, Morrison CE. Waist circumference as a measure for indicating need for weight management. BMJ. 1995;311:158–61.
Sluik D, Boeing H, Montonen J, Pischon T, Kaaks R, Teucher B, et al. Associations between general and abdominal adiposity and mortality in individuals with diabetes mellitus. Am J Epidemiol. 2011;174:22–34.
Kuk JL, Janiszewski PM, Ross R. Body mass index and hip and thigh circumferences are negatively associated with visceral adipose tissue after control for waist circumference. Am J Clin Nutr. 2007;85:1540–4.
Acknowledgements
We thank the staff and the participants of the UK Biobank study. This research has been conducted using the UK Biobank Resource (application 37142).
Funding
SR was supported by project grants from the Swedish Research Council (Vetenskapsrådet [VR], 2016-01527), the Swedish state under the agreement between the Swedish government and the county councils (the ALF agreement) (SU 2018-04276), the Swedish Diabetes Foundation (DIA 2017-205), the Swedish Heart-Lung Foundation (20120533), the Wallenberg Academy Fellows from the Knut and Alice Wallenberg Foundation (KAW 2017.0203), the Astra Zeneca Agreement for Research, Grant SSF ITM17-0384 Swedish Foundation for Strategic Research and Novo Nordisk Project Grants in Endocrinology & Metabolism - Nordic Region 2020. LV was supported by MyFirst Grant AIRC n.16888, Ricerca Finalizzata Ministero della Salute RF-2016-02364358, Ricerca corrente Fondazione IRCCS Ca’ Granda Ospedale Maggiore Policlinico, the European Union (EU) Programme Horizon 2020 (under grant agreement No. 777377) for the project LITMUS- “Liver Investigation: Testing Marker Utility in Steatohepatitis”, Fondazione IRCCS Ca’ Granda “Liver BIBLE” PR-0391, Fondazione IRCCS Ca’ Granda core COVID-19 Biobank (RC100017A).
Author information
Authors and Affiliations
Contributions
SR and UVG contributed to study concept and design. ADV, SR, and UVG contributed to drafting the manuscript. ADV contributed to perform the statistical analyses. All authors contributed to analysis and interpretation of data, critically revised the manuscript for important intellectual content, and approved the final version for submission. All authors had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Corresponding authors
Ethics declarations
Competing interests
SR has served as a consultant for AstraZeneca, Celgene, Sanofi, Amgen, Akcea Therapeutics, Camp4, Medacorp, and Ambys in the last 3 years. SR has received research grants from AstraZeneca in the last 2 years. The others declare no financial or other relationships with drug manufacturers that could lead to a conflict of interest.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
De Vincentis, A., Tavaglione, F., Spagnuolo, R. et al. Metabolic and genetic determinants for progression to severe liver disease in subjects with obesity from the UK Biobank. Int J Obes 46, 486–493 (2022). https://doi.org/10.1038/s41366-021-01015-w
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41366-021-01015-w
This article is cited by
-
Prognostication in NAFLD: physiological bases, clinical indicators, and newer biomarkers
Journal of Physiology and Biochemistry (2023)
-
The link between liver fat and cardiometabolic diseases is highlighted by genome-wide association study of MRI-derived measures of body composition
Communications Biology (2022)