Abstract
The number of cancers attributed to obesity is increasing over time. The mechanisms classically implicated in cancer pathogenesis and progression in patients with obesity involve adiposity-related alteration of insulin, sex hormones, and adipokine pathways. However, they do not fully capture the complexity of the association between obesity-related nutritional imbalance and cancer. Gut hormones are secreted by enteroendocrine cells along the gastrointestinal tract in response to nutritional cues, and act as nutrient sensors, regulating eating behavior and energy homeostasis and playing a role in immune-modulation. The dysregulation of gastrointestinal hormone physiology has been implicated in obesity pathogenesis. For their peculiar function, at the cross-road between nutrients intake, energy homeostasis and inflammation, gut hormones might represent an important but still underestimated mechanism underling the obesity-related high incidence of cancer. In addition, cancer research has revealed the widespread expression of gut hormone receptors in neoplastic tissues, underscoring their implication in cell proliferation, migration, and invasion processes that characterize tumor growth and aggressiveness. In this review, we hypothesize that obesity-related alterations in gut hormones might be implicated in cancer pathogenesis, and provide evidence of the pathways potentially involved.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others
References
Song M, Giovannucci E. Estimating the influence of obesity on cancer risk: stratification by smoking is critical. J Clin Oncol. 2016;34:3237–9.
Pollak M. Do cancer cells care if their host is hungry? Cell Metab. 2009;9:401–3.
Makaronidis JM, Battheram RL. The role of gut hormones in the pathogenesis and management of obesity. Curr Opin Physiol. 2019;12:1–11.
Steinert R, Feinle-Bisset C, Asarian L, Horowitz M, Beglinger C, Geary N, et al. GLP-1, and PYY(3-36): secretory controls and physiological roles in eating and glycemia in health, obesity, and after RYGB. Physiol Rev. 2017;97:411–63.
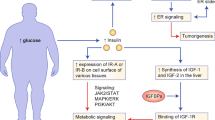
Johnson JA, Carstensen B, Witte D, Bowker SL, Lipscombe L, Renehan AG. Diabetes and Cancer Research Consortium Diabetes and cancer (1): evaluating the temporal relationship between type 2 diabetes and cancer incidence. Diabetologia.2012;55:1607–18.
Michael P. Insulin and insulin-like growth factor signalling in neoplasia. Nat Rev Cancer. 2008;8:915–28.
Van Kruijsdijk RCM, van der Wall E, Visseren FLJ. Obesity and cancer: the role of dysfunctional adipose tissue. Cancer Epidemiol Biomarkers Prev. 2009;18:2569–78.
Ryan Kolb R, Sutterwala FS, Zhang W. Obesity and cancer: inflammation bridges the two. Curr Opin Pharmacol. 2016;29:77–89.
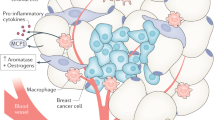
Picon-Ruiz M, Pan C, Drews-Elger K, Jang K, Besser AH, Zhao D, et al. Interactions between adipocytes and breast cancer cells stimulate cytokine production and drive Src/Sox2/miR-302b-mediated malignant progression. Cancer Res. 2016;76:491–504.
Subbaramaiah K, Morris PG, Zhou XK, Morrow M, Du B, Giri D, Kopelovich L, et al. Increased levels of COX-2 and prostaglandin E2 contribute to elevated aromatase expression in inflamed breast tissue of obese women. Cancer Discov. 2012;2:356–65.
Terzić J, Grivennikov S, Karin E, Karin M. Inflammation and colon cancer. Gastroenterology. 2010;138:2101–2114.e5.
Moossavi S, Bishehsari F. Inflammation in sporadic colorectal cancer. Arch Iran Med. 2012;15:166–70.
Taniguchi K, Karin M. IL-6 and related cytokines as the critical lynchpins between inflammation and cancer. Semin Immunol. 2014;26:54–74.
Liu Z, Ryan S, Brooks RS, Ciappio ED, Kim SJ, Crott JW, et al. Diet-induced obesity elevates colonic TNF-α in mice and is accompanied by an activation of Wnt signaling: a mechanism for obesity-associated colorectal cancer. J Nutr Biochem. 2012;23:1207–13.
Neuschwander‐Tetri BA. Hepatic lipotoxicity and the pathogenesis of nonalcoholic steatohepatitis: the central role of nontriglyceride fatty acid metabolites. Hepatology. 2010;52:774–88.
Nakagawa H, Umemura A, Taniguchi K, Font-Burgada J, Dhar D, Ogata H, et al. ER stress cooperates with hypernutrition to trigger TNF-dependent spontaneous HCC development. Cancer Cell. 2014;26:331–43.
Park JH, Yu GY, He G, Ali SR, Holzer RG, et al. Dietary and genetic obesity promote liver inflammation and tumorigenesis by enhancing IL-6 and TNF expression. Clinical Trial. Cancer Res. 2010;140:197–208.
Lavalette C, Adjibade M, Srour M, Sellem L, Fiolet T, Hercberg S, et al. Cancer-specific and general nutritional scores and cancer risk: results from the prospective NutriNet-Santé Cohort. Cancer Res. 2018;78:4427–35.
Ruiz-Canela M, Estruch R, Corella D, Salas-Salvadó J, Martínez-González MA. Association of Mediterranean diet with peripheral artery disease: the PREDIMED Randomized Trial. JAMA. 2014;311:415–7.
Pierce JP, Natarajan L, Caan BJ, Parker BA, Greenberg ER, Flatt SW, et al. Influence of a diet very high in vegetables, fruit, and fiber and low in fat on prognosis following treatment for breast cancer: the Women’s Healthy Eating and Living (WHEL) randomized trial. Clinical Trial. Nutrition. 2007;298:289–98.
Fine EJ, Segal-Isaacson CJ, Feinman RD, Herszkopf S, Romano MC, Tomuta N, et al. Targeting insulin inhibition as a metabolic therapy in advanced cancer: a pilot safety and feasibility dietary trial in 10 patients. Cell Metab. 2012;28:1028–35.
Malik S, McGlone F, Bedrossian D, Dagher A. Ghrelin modulates brain activity in areas that control appetitive behavior. J Mol Endocrinol. 2008;7:400–9.
Naznin F, Toshinai K, Waise TMZ, Okada T, Sakoda H, Nakazato M. Restoration of metabolic inflammation-related ghrelin resistance by weight loss. Review trends. Neuroscience. 2018;60:109–18.
Al Massadi O, López M, Tschöp M, Diéguez C, Nogueiras R. Current understanding of the hypothalamic ghrelin pathways inducing appetite and adiposity. Randomized controlled trial. Cell Metab. 2017;40:167–80.
De Silva A, Salem V, Long CJ, Makwana A, Newbould RD, Rabiner EA, et al. The gut hormones PYY 3-36 and GLP-1 7-36 amide reduce food intake and modulate brain activity in appetite centers in humans. Cell Metab. 2011;14:700–6.
Holst JJ. The physiology of glucagon-like peptide 1. Physiol Rev. 2007;87:1409–39.
Nauck MA, Bartels E, Orskov C, Ebert R, Creutzfeldt W. Additive insulinotropic effects of exogenous synthetic human gastric inhibitory polypeptide and glucagon-like peptide-1-(7-36) amide infused at near-physiological insulinotropic hormone and glucose concentrations. J Clin Endocrinol Metab. 1993;76:912–7.
Dockray CJ. Cholecystokinin. Curr Opin Endocrinol Diabetes Obes. 2012;19:8–12.
Brown JA, Bugescu R, Mayer TA, Gata-Garcia A, Kurt G, Woodworth HL, et al. Loss of action via neurotensin-leptin receptor neurons disrupts leptin and ghrelin-mediated control of energy balance. Endocrinology. 2017;158:1271–88.
Grunddal KV, Ratner CF, Svendsen B, Sommer F, Engelstoft MS, Madsen AN, et al. Neurotensin is coexpressed, coreleased, and acts together with GLP-1 and PYY in enteroendocrine control of metabolism. Endocrinology. 2016;157:176–94.
Barchetta I, Cimini FA, Capoccia D, Bertoccini L, Ceccarelli V, Chiappetta C, et al. Neurotensin is a lipid-induced gastrointestinal peptide associated with visceral adipose tissue inflammation in obesity. Nutrients. 2018;10:526.
Pereira JADS, Da Silva FC, De Moraes-Vieira PMM. The impact of ghrelin in metabolic diseases: an immune perspective. J Diabetes Res. 2017;2017:4527980.
Tschöp M, Weyer C, Tataranni PA, Devanarayan V, Ravussin E, Heiman ML. Circulating ghrelin levels are decreased in human obesity. Diabetes. 2001;50:707–9.
Takagi K, Legrand R, Asakawa A, Amitani H, François M, Tennoune N, et al. Anti-ghrelin immunoglobulins modulate ghrelin stability and its orexigenic effect in obese mice and humans. Nat Commun. 2013;4:2685.
Al-Dwairi A, Alqudah TE, Al-Shboul O, Alqudah M, Mustafa AG, Alfaqih MA. Glucagon-like peptide-1 exerts anti-inflammatory effects on mouse colon smooth muscle cells through the cyclic adenosine monophosphate/nuclear factor-κB pathway in vitro. Inflamm Res. 2018;11:95–109.
Larsson LI, Fahrenkrug J, Schaffalitzky De Muckadell O, Sundler F, Hakanson R, Rehfeld JR. Localization of vasoactive intestinal polypeptide (VIP) to central and peripheral neurons. Proc Natl Acad Sci USA. 1976;73:3197–200.
Iwasaki M, Akiba Y, Kaunitz JD. Recent advances in vasoactive intestinal peptide physiology and pathophysiology: focus on the gastrointestinal system. F1000Res. 2019;8:1629.
Ganea D, Hooper KM, Kong W. The neuropeptide vasoactive intestinal peptide: direct effects on immune cells and involvement in inflammatory and autoimmune diseases. Acta Physiol (Oxf). 2015;213:442–52.
Ganea D, Rodriguez R, Delgado M. Vasoactive intestinal peptide and pituitary adenylate cyclase-activating polypeptide: players in innate and adaptive immunity. Cell Mol Biol (Noisy-le-Grand, France). 2003;49:127–42.
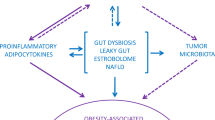
Lay RE, Turnbaugh PJ, Klein S, Gordon JI. Microbial ecology: human gut microbes associated with obesity. Nature. 2006;444:1022–3.
Bergman EN. Energy contributions of volatile fatty acids from the gastrointestinal tract in various species. Physiol Rev. 1990;70:567–90.
Everard A, Cani PD. Gut microbiota and GLP-1. Rev Endocr Metab Disord. 2014;15:189–96.
Zheng W, Zhao W, Wu M, Song X, Caro F, Sun X, et al. Microbiota-targeted maternal antibodies protect neonates from enteric infection. Nature. 2020;577:543–8.
Christ A, Lauterbach M, Latz E. Western diet and the immune system: an inflammatory connection. Immunity. 2019;51:794–811.
Cheng L, Jin H, Qiang Y, Wu S, Yan C, Han M, et al. High fat diet exacerbates dextran sulfate sodium induced colitis through disturbing mucosal dendritic cell homeostasis. Int Immunopharmacol. 2016;40:1–10.
Arpaia N, Campbell C, Fan X, Dikiy S, van der Veeken J, deRoos P, et al. Metabolites produced by commensal bacteria promote peripheral regulatory T-cell generation Nature. 2013;504:451–5.
Rodriguez-Palacios A, Harding A, Menghini P, Himmelman C, Retuerto M, Nickerson KP, et al. The artificial sweetener splenda promotes gut proteobacteria, dysbiosis, and myeloperoxidase reactivity in Crohn’s disease-like ileitis. Inflamm Bowel Dis. 2018;24:1005–20.
Zaami S. New psychoactive substances: concerted efforts and common legislative answers for stemming a growing health hazard. Eur Rev Med Pharmacol Sci. 2019;239681–90.
Truax AD, Chen L, Tam JW, Cheng N, Guo H, Koblansky AA, et al. The inhibitory innate immune sensor NLRP12 maintains a threshold against obesity by regulating gut microbiota homeostasis. Cell Host Microbe. 2018;24:364–78.
Bains M, Laney C, Wolfe AE, Orr M, Waschek JA, Ericsson AC, et al. Vasoactive intestinal peptide deficiency is associated with altered gut microbiota communities in male and female C57BL/6 mice. Front Microbiol. 2019;10:2689.
Matson V, Fessler J, Bao R, Chongsuwat T, Zha Y, Alegre ML, et al. The commensal microbiome is associated with anti-PD-1 efficacy in metastatic melanoma patients. Science. 2018. 2018;359:104–8.
Gopalakrishnan V, Spencer CN, Nezi L, Reuben A, Andrews MC, Karpinets TV, et al. Gut microbiome modulates response to anti-PD-1 immunotherapy in melanoma patients. Science. 2018;359:97–103.
Sivan A, Corrales L, Hubert N, Williams JB, Aquino-Michaels K, Earley ZM, et al. Commensal Bifidobacterium promotes antitumor immunity and facilitates anti-PD-L1 efficacy. Science. 2015;350:1084–9.
Routy B, Le Chatelier E, Derosa L, Duong CPM, Alou MT, Daillère R, et al. Gut microbiome influences efficacy of PD-1-based immunotherapy against epithelial tumors. Science. 2018;359:91–7.
Thomas RP, Hellmich MR, Townsend CM Jr, Evers BM. Role of gastrointestinal hormones in the proliferation of normal and neoplastic tissues. Endocr Rev. 2003;24:571–99.
Faubert B, Solmonson A, De Berardinis RJ. Metabolic reprogramming and cancer progression. Science. 2020;368:eaaw5473.
Maoret JJ, Anini Y, Rouyer-Fessard C, Gully D, Laburthe M. Neurotensin and a non-peptide neurotensin receptor antagonist control human colon cancer cell growth in cell culture and in cells xenografted into nude mice. Int J Cancer. 1999;80:448–54.
Gui X, Guzman G, Dobner PR, Kadkol SS. Increased neurotensin receptor-1 expression during progression of colonic adenocarcinoma. Peptides. 2008;29:1609–15.
Akter H, Yoon JH, Yoo YS, Kang MJ. Validation of neurotensin receptor 1 as a therapeutic target for gastric cancer. Mol Cells. 2018;41:591–602.
Younes M, Wu Z, Dupouy S, Lupo AM, Mourra N, Takahashi T, et al. Neurotensin (NTS) and its receptor (NTSR1) causes EGFR, HER2 and HER3 over-expression and their autocrine/paracrine activation in lung tumors, confirming responsiveness to erlotinib. Oncotarget. 2014;5:8252–69.
Moody TW, Chan DC, Mantey SA, Moreno P, Jensen RT. SR48692 inhibits non-small cell lung cancer proliferation in an EGF receptor-dependent manner. Life Sci. 2014;100:25–34.
Alifano M, Souaze F, Dupouy S, Camilleri-Broet S, Younes M, Ahmed-Zaid SM, et al. Neurotensin receptor 1 determines the outcome of non-small cell lung cancer. Clin Cancer Res. 2010;16:4401–10.
Dupouy S, Doan VK, Wu Z, Mourra N, Liu J, De Wever O, et al. Activation of EGFR, HER2 and HER3 by neurotensin/neurotensin receptor 1 renders breast tumors aggressive yet highly responsive to lapatinib and metformin in mice. Oncotarget. 2014;5:8235–51.
Morgat C, Chastel A, Molinie V, Schollhammer R, Macgrogan G, Velasco V, et al. Neurotensin receptor-1 expression in human prostate cancer: a pilot study on primary tumors and lymph node metastases. Int J Mol Sci. 2019;20:1721.
Zhu S, Tian H, Niu X, Wang J, Li X, Jiang N, et al. Neurotensin and its receptors mediate neuroendocrine transdifferentiation in prostate cancer. Oncogene. 2019;38:4875–84.
DaSilva JO, Amorino GP, Casarez EV, Pemberton B, Parsons SJ. Neuroendocrine-derived peptides promote prostate cancer cell survival through activation of IGF-1R signaling. Prostate. 2013;73:801–12.
Prabakaran D, Wang B, Feuerstein JD, Sinclair JA, Bijpuria P, Jepeal LI, et al. Glucose-dependent insulinotropic polypeptide stimulates the proliferation of colorectal cancer cells. Regul Pept. 2010;163:74–80.
Waser B, Rehmann R, Sanchez C, Fourmy D, Reubi JC. Glucose-dependent insulinotropic polypeptide receptors in most gastroenteropancreatic and bronchial neuroendocrine tumors. J Clin Endocrinol Metab. 2012;97:482–8.
Korner M, Waser B, Reubi JC. Does somatostatin or gastric inhibitory peptide receptor expression correlate with tumor grade and stage in gut neuroendocrine tumors? Neuroendocrinology. 2015;101:45–57.
Patel YC. Somatostatin and its receptor family. Front Neuroendocrinol. 1999;20:157–98.
Caplin ME, Pavel M, Cwikla JB, Phan AT, Raderer M, Sedlackova E, et al. Anti-tumour effects of lanreotide for pancreatic and intestinal neuroendocrine tumours: the CLARINET open-label extension study. Endocr Relat Cancer. 2016;23:191–9.
Rinke A, Muller HH, Schade-Brittinger C, Klose KJ, Barth P, Wied M, et al. Placebo-controlled, double-blind, prospective, randomized study on the effect of octreotide LAR in the control of tumor growth in patients with metastatic neuroendocrine midgut tumors: a report from the PROMID Study Group. J Clin Oncol. 2009;27:4656–63.
Colao A, Auriemma RS, Pivonello R. The effects of somatostatin analogue therapy on pituitary tumor volume in patients with acromegaly. Pituitary. 2016;19:210–21.
Mukhina S, Liu D, Guo K, Raccurt M, Borges-Bendris S, Mertani HC, et al. Autocrine growth hormone prevents lactogenic differentiation of mouse mammary epithelial cells. Endocrinology. 2006;147:1819–29.
Villa-Osaba A, Gahete MD, Cordoba-Chacon J, de Lecea L, Pozo-Salas AI, Delgado-Lista FJ, et al. Obesity alters gene expression for GH/IGF-I axis in mouse mammary fat pads: differential role of cortistatin and somatostatin. PLoS One. 2015;10:e0120955.
Luque RM, Villa-Osaba A,FLL, Pozo-Salas AI, Sanchez-Sanchez R, Ortega-Salas R, et al. Lack of cortistatin or somatostatin differentially influences DMBA-induced mammary gland tumorigenesis in mice in an obesity-dependent mode. Breast Cancer Res. 2016;18:29.
Smith JP, Kramer ST, Solomon TE. CCK stimulates growth of six human pancreatic cancer cell lines in serum-free medium. Regul Pept. 1991;32:341–9.
Williams JA. Intracellular signaling mechanisms activated by cholecystokinin-regulating synthesis and secretion of digestive enzymes in pancreatic acinar cells. Annu Rev Physiol. 2001;63:77–97.
Nadella S, Burks J, Al-Sabban A, Inyang G, Wang J, Tucker RD, et al. Dietary fat stimulates pancreatic cancer growth and promotes fibrosis of the tumor microenvironment through the cholecystokinin receptor. Am J Physiol Gastrointest Liver Physiol. 2018;315:G699–G712.
McWilliams DF, Watson SA, Crosbee DM, Michaeli D, Seth R. Coexpression of gastrin and gastrin receptors (CCK-B and delta CCK-B) in gastrointestinal tumour cell lines. Gut. 1998;42:795–8.
Tang KD, Liu J, Jovanovic L, An J, Hill MM, Vela I, et al. Adipocytes promote prostate cancer stem cell self-renewal through amplification of the cholecystokinin autocrine loop. Oncotarget. 2016;7:4939–48.
Buteau J, El-Assaad W, Rhodes CJ, Rosenberg L, Joly E, Prentki M. Glucagon-like peptide-1 prevents beta cell glucolipotoxicity. Diabetologia. 2004;47:806–15.
Farilla L, Bulotta A, Hirshberg B, Li Calzi S, Khoury N, Noushmehr H, et al. Glucagon-like peptide 1 inhibits cell apoptosis and improves glucose responsiveness of freshly isolated human islets. Endocrinology. 2003;144:5149–58.
Koehler JA, Drucker DJ. Activation of glucagon-like peptide-1 receptor signaling does not modify the growth or apoptosis of human pancreatic cancer cells. Diabetes. 2006;55:1369–79.
Pinto LC, Falcetta MR, Rados DV, Leitao CB, Gross JL. Glucagon-like peptide-1 receptor agonists and pancreatic cancer: a meta-analysis with trial sequential analysis. Sci Rep. 2019;9:2375.
Vangoitsenhoven R, Mathieu C, Van der Schueren B. GLP1 and cancer: friend or foe? Endocr Relat Cancer. 2012;19:F77–88.
Nauck MA, Jensen TJ, Rosenkilde C, Calanna S, Buse JB. LEADER Publication Committee on behalf of the LEADER Trial Investigators Neoplasms reported with liraglutide or placebo in people with type 2 diabetes: results from the LEADER randomized trial. Diabetes Care. 2018;41:1663–71.
Bjerre Knudsen L, Madsen LW, Andersen S, Almholt K, de Boer AS, Drucker DJ, et al. Glucagon-like Peptide-1 receptor agonists activate rodent thyroid C-cells causing calcitonin release and C-cell proliferation. Endocrinology. 2010;151:1473–86.
Fidan-Yaylali G, Dodurga Y, Secme M, Elmas L. Antidiabetic exendin-4 activates apoptotic pathway and inhibits growth of breast cancer cells. Tumour Biol. 2016;37:2647–53.
Luciani P, Deledda C, Benvenuti S, Squecco R, Cellai I, Fibbi B, et al. Exendin-4 induces cell adhesion and differentiation and counteracts the invasive potential of human neuroblastoma cells. PLoS One. 2013;8:e71716.
Kanda R, Hiraike H, Wada-Hiraike O, Ichinose T, Nagasaka K, Sasajima Y, et al. Expression of the glucagon-like peptide-1 receptor and its role in regulating autophagy in endometrial cancer. BMC Cancer. 2018;18:657.
Sever S, White DL, Garcia JM. Is there an effect of ghrelin/ghrelin analogs on cancer? A systematic review. Endocr Relat Cancer. 2016;23:R393–409.
Fung JN, Seim I, Wang D, Obermair A, Chopin LK, Chen C. Expression and in vitro functions of the ghrelin axis in endometrial cancer. Horm Cancer. 2010;1:245–55.
Yeh AH, Jeffery PL, Duncan RP, Herington AC, Chopin LK. Ghrelin and a novel preproghrelin isoform are highly expressed in prostate cancer and ghrelin activates mitogen-activated protein kinase in prostate cancer. Clin Cancer Res. 2005;11:8295–303.
Murphy G, Kamangar F, Dawsey SM, Stanczyk FZ, Weinstein SJ, Taylor PR, et al. The relationship between serum ghrelin and the risk of gastric and esophagogastric junctional adenocarcinomas. J Natl Cancer Inst. 2011;103:1123–9.
D’Onghia V, Leoncini R, Carli R, Santoro A, Giglioni S, Sorbellini F, et al. Circulating gastrin and ghrelin levels in patients with colorectal cancer: correlation with tumour stage, Helicobacter pylori infection and BMI. Biomed Pharmacother. 2007;61:137–41.
Murphy G, Cross AJ, Dawsey SM, Stanczyk FZ, Kamangar F, Weinstein SJ, et al. Serum ghrelin is associated with risk of colorectal adenocarcinomas in the ATBC study. Gut. 2018;67:1646–51.
Docanto MM, Yang F, Callaghan B, Au CC, Ragavan R, Wang X, et al. Ghrelin and des-acyl ghrelin inhibit aromatase expression and activity in human adipose stromal cells: suppression of cAMP as a possible mechanism. Breast Cancer Res Treat. 2014;147:193–201.
Gronberg M, Fjallskog ML, Jirstrom K, Janson ET. Expression of ghrelin is correlated to a favorable outcome in invasive breast cancer. Acta Oncol. 2012;51:386–93.
Soleyman-Jahi S, Sadeghi F, Pastaki Khoshbin A, Khani L, Roosta V, Zendehdel K. Attribution of ghrelin to cancer; attempts to unravel an apparent controversy. Front Oncol. 2019;9:1014.
Liu CD, Slice LW, Balasubramaniam A, Walsh JH, Newton TR, Saxton RE, et al. Y2 receptors decrease human pancreatic cancer growth and intracellular cyclic adenosine monophosphate levels. Surgery. 1995;118:229–35.
Adrian TE, Ballantyne GH, Zucker KA, Zdon MJ, Tierney R, Modlin IM. Lack of peptide YY immunoreactivity in adenomatous colonic polyps: evidence in favor of an adenoma-carcinoma sequence. J Surg Res. 1988;44:561–5.
Sgambati SA, Turowski GA, Basson MD. Peptide YY selectively stimulates expression of the colonocytic phenotype. J Gastrointest Surg. 1997;1:561–8.
Kling K, Kim F, Cole M, McFadden D. B-cell leukemia protein-2 and peptide YY chemotherapy resistance in colon cancer. Am J Surg. 1999;178:411–4.
Liu CD, Balasubramaniam A, Saxton RE, Paiva M, McFadden DW. Human pancreatic cancer growth is inhibited by peptide YY and BIM-43004-1. J Surg Res. 1995;58:707–12.
Grise KR, Rongione AJ, Laird EC, McFadden DW. Peptide YY inhibits growth of human breast cancer in vitro and in vivo. J Surg Res. 1999;82:151–5.
Heisler T, Towfigh S, Simon N, McFadden DW. Peptide YY and vitamin E inhibit hormone-sensitive and -insensitive breast cancer cells. J Surg Res. 2000;91:9–14.
Moody TW, Nuche-Berenguer B, Jensen RT. Vasoactive intestinal peptide/pituitary adenylate cyclase activating polypeptide, and their receptors and cancer. Curr Opin Endocrinol Diabetes Obes. 2016;23:38–47.
Liu S, Zeng Y, Li Y, Guo W, Liu J, Ouyang N. VPAC1 overexpression is associated with poor differentiation in colon cancer. Tumour Biol. 2014;35:6397–404.
Levy A, Gal R, Granoth R, Dreznik Z, Fridkin M, Gozes I. In vitro and in vivo treatment of colon cancer by VIP antagonists. Regul Pept. 2002;109:127–33.
Valdehita A, Bajo AM, Schally AV, Varga JL, Carmena MJ, Prieto JC. Vasoactive intestinal peptide (VIP) induces transactivation of EGFR and HER2 in human breast cancer cells. Mol Cell Endocrinol. 2009;302:41–8.
Zia H, Hida T, Jakowlew S, Birrer M, Gozes Y, Reubi JC, et al. Breast cancer growth is inhibited by vasoactive intestinal peptide (VIP) hybrid, a synthetic VIP receptor antagonist. Cancer Res. 1996;56:3486–9.
Garcia-Fernandez MO, Solano RM, Carmena MJ, Busto R, Bodega G, Ruiz-Villaespesa A, et al. Expression of functional PACAP/VIP receptors in human prostate cancer and healthy tissue. Peptides. 2003;24:893–902.
Fernandez-Martinez AB, Carmena MJ, Bajo AM, Vacas E, Sanchez-Chapado M, Prieto JC. VIP induces NF-kappaB1-nuclear localisation through different signalling pathways in human tumour and non-tumour prostate cells. Cell Signal. 2015;27:236–44.
Acknowledgements
Obesity Programs of Nutrition, Education, Research and Assessment (OPERA) group members: Annamaria Colao, Carlo Alviggi, Sara Aprano, Rocco Barazzoni, Luigi Barrea, Francesco Beguinot, Annamaria Belfiore, Giuseppe Bellastella, Silvia Bettini, Giuseppe Bifulco, Maurizio Bifulco, Caterina Brasacchio, Filomena Bottiglieri, Luca Busetto, Brunella Capaldo, Massimiliano Caprio, Felipe Casanueva, Luigi Di Luigi, Andrea Di Nisio, Laura Di Renzo, Carolina Di Somma, Lorenzo Maria Donini, Katherine Esposito, Massimo Federici, Dario Giugliano, Lucio Gnessi, Gianluca Gortan Cappellari, Brunella Guida, Maria Angela Guzzardi, Daniela Laudisio, Andrea Lenzi, Alessia Liccardi, Carla Lubrano, Paolo Emidio Macchia, Silvia Magno, Paolo Marzullo, Davide Menafra, Silvia Migliaccio, Fabrizio Muratori, Giovanna Muscogiuri, Raffaele Napoli, Caterina Pelosini, Francesca Pivari, Rosario Pivonello, Eleonora Poggiogalle, Gabriella Pugliese, Gabriele Riccardi, Alberto Ritieni, Fiammetta Romano, Domenico Salvatore, Alessandro Sanduzzi, Ferruccio Santini, Silvia Savastano, Paolo Sbraccia, Giovanni Scambia Laura Soldati, Giovanni Spera, Maria Grazia Tarsitano, Dario Tuccinardi, Olga Vaccaro, Mary Venneri, Samir Sukkar, and Roberto Vettor.
Funding
This article is published as part of a supplement funded by the scientific assistance of Panta Rei Impresa Sociale srl (https://www.panta-rei.eu/pantarei/).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Guzzardi, M.A., Pugliese, G., Bottiglieri, F. et al. Obesity-related gut hormones and cancer: novel insight into the pathophysiology. Int J Obes 45, 1886–1898 (2021). https://doi.org/10.1038/s41366-021-00865-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41366-021-00865-8