Abstract
Background
Obesity fosters worse clinical outcomes in both premenopausal and postmenopausal women with breast cancer. Emerging evidence suggests that an android body fat distribution in particular is deleterious for breast cancer prognosis. The extent of adipose tissue dysfunction, especially how it relates to breast cancer prognostic factors and anthropometric measurements, has not been fully investigated.
Objective
Our objective was to examine if markers of adipose tissue dysfunction, such as hypertrophy and macrophage accumulation, are relevant for the pathophysiology of breast cancer and its associated prognostic factors in a well-characterised cohort of women with breast cancer who did not receive treatment before surgery.
Methods
A consecutive series of 164 women with breast cancer provided breast adipose tissue sample. Multivariate generalised linear models were used to test associations of anthropometric indices and prognostic factors with markers of adipose tissue dysfunction.
Results
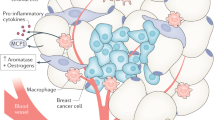
We found associations of breast adipocyte size and macrophage infiltration (number of CD68+ cells/100 adipocytes) with adiposity, particularly a strong association between breast adipocyte size and central obesity, independent of total adiposity, age and menopausal status (βadj = 0.87; p = 0.0001). We also identified relationships of adipocyte hypertrophy and macrophage infiltration with prognostic factors, such as cancer stage and tumour grade (p < 0.05). RNA expression of pro-inflammatory cytokines (IL6, TNF) and leptin was also increased as a function of adipocyte size and CD86+/CD11c+ macrophage number/100 adipocytes (p < 0.05).
Conclusions
Our findings support the model of dysfunctional adipose tissue in obesity-associated breast cancer.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others
References
Calle EE, Rodriguez C, Walker-Thurmond K, Thun MJ. Overweight, obesity, and mortality from cancer in a prospectively studied cohort of U.S. adults. N Engl J Med. 2003;348:1625–38.
Widschwendter P, Friedl TW, Schwentner L, DeGregorio N, Jaeger B, Schramm A, et al. The influence of obesity on survival in early, high-risk breast cancer: results from the randomized SUCCESS A trial. Breast Cancer Res. 2015;17:129.
Cho WK, Choi DH, Park W, Cha H, Nam SJ, Kim SW, et al. Effect of body mass index on survival in breast cancer patients according to subtype, metabolic syndrome, and treatment. Clin Breast Cancer. 2018;18:e1141–7.
Biganzoli E, Desmedt C, Fornili M, de Azambuja E, Cornez N, Ries F, et al. Recurrence dynamics of breast cancer according to baseline body mass index. Eur J Cancer. 2017;87:10–20.
Copson ER, Cutress RI, Maishman T, Eccles BK, Gerty S, Stanton L, et al. Obesity and the outcome of young breast cancer patients in the UK: the POSH study. Ann Oncol. 2015;26:101–12.
Tchernof A, Després JP. Pathophysiology of human visceral obesity: an update. Physiol Rev. 2013;93:359–404.
George SM, Bernstein L, Smith AW, Neuhouser ML, Baumgartner KB, Baumgartner RN, et al. Central adiposity after breast cancer diagnosis is related to mortality in the Health, Eating, Activity, and Lifestyle study. Breast Cancer Res Treat. 2014;146:647–55.
Abrahamson PE, Gammon MD, Lund MJ, Flagg EW, Porter PL, Stevens J, et al. General and abdominal obesity and survival among young women with breast cancer. Cancer Epidemiol Biomarkers Prev. 2006;15:1871–7.
Russo L, Lumeng CN. Properties and functions of adipose tissue macrophages in obesity. Immunology. 2018;155:407–17.
Laforest S, Labrecque J, Michaud A, Cianflone K, Tchernof A. Adipocyte size as a determinant of metabolic disease and adipose tissue dysfunction. Crit Rev Clin Lab Sci. 2015;52:301–13.
Blüher M. Adipose tissue dysfunction in obesity. Exp Clin Endocrinol Diabetes. 2009;117:241–50.
Michaud A, Tordjman J, Pelletier M, Liu Y, Laforest S, Noël S, et al. Relevance of omental pericellular adipose tissue collagen in the pathophysiology of human abdominal obesity and related cardiometabolic risk. Int J Obes. 2016;40:1823–31.
Divoux A, Tordjman J, Lacasa D, Veyrie N, Hugol D, Aissat A, et al. Fibrosis in human adipose tissue: composition, distribution, and link with lipid metabolism and fat mass loss. Diabetes. 2010;59:2817–25.
Morris PG, Hudis CA, Giri D, Morrow M, Falcone DJ, Zhou XK, et al. Inflammation and increased aromatase expression occur in the breast tissue of obese women with breast cancer. Cancer Prev Res. 2011;4:1021–9.
Iyengar NM, Morris PG, Zhou XK, Gucalp A, Giri D, Harbus MD, et al. Menopause is a determinant of breast adipose inflammation. Cancer Prev Res. 2015;8:349–58.
Hanna M, Dumas I, Jacob S, Têtu B, Diorio C. Physical activity, mammographic density, and age-related lobular involution among premenopausal and postmenopausal women. Menopause. 2015;22:964–75.
Laforest S, Pelletier M, Michaud A, Daris M, Descamps J, Soulet D, et al. Histomorphometric analyses of human adipose tissues using intact, flash-frozen samples. Histochem Cell Biol. 2018;149:209–18.
Ghosh K, Vachon CM, Pankratz VS, Vierkant RA, Anderson SS, Brandt KR, et al. Independent association of lobular involution and mammographic breast density with breast cancer risk. J Natl Cancer Inst. 2010;102:1716–23.
Brown KA, Iyengar NM, Zhou XK, Gucalp A, Subbaramaiah K, Wang H, et al. Menopause is a determinant of breast aromatase expression and its associations with BMI, inflammation, and systemic markers. J Clin Endocrinol Metab. 2017;102:1692–701.
Vaysse C, Lomo J, Garred O, Fjeldheim F, Lofteroed T, Schlichting E, et al. Inflammation of mammary adipose tissue occurs in overweight and obese patients exhibiting early-stage breast cancer. NPJ Breast Cancer. 2017;3:19.
Pouliot MC, Després JP, Lemieux S, Moorjani S, Bouchard C, Tremblay A, et al. Waist circumference and abdominal sagittal diameter: best simple anthropometric indexes of abdominal visceral adipose tissue accumulation and related cardiovascular risk in men and women. Am J Cardiol. 1994;73:460–8.
Iyengar NM, Zhou XK, Gucalp A, Morris PG, Howe LR, Giri DD, et al. Systemic correlates of white adipose tissue inflammation in early-stage breast cancer. Clin Cancer Res. 2016;22:2283–9.
Hanna M, Dumas I, Orain M, Jacob S, Têtu B, Diorio C. Association between physical activity and the expression of mediators of inflammation in normal breast tissue among premenopausal and postmenopausal women. Cytokine. 2018;102:151–60.
Harwani SC. Macrophages under pressure: the role of macrophage polarization in hypertension. Transl Res. 2018;191:45–63.
Veilleux A, Rhéaume C, Daris M, Luu-The V, Tchernof A. Omental adipose tissue type 1 11 beta-hydroxysteroid dehydrogenase oxoreductase activity, body fat distribution, and metabolic alterations in women. J Clin Endocrinol Metab. 2009;94:3550–7.
Veilleux A, Laberge PY, Morency J, Noël S, Luu-The V, Tchernof A. Expression of genes related to glucocorticoid action in human subcutaneous and omental adipose tissue. J Steroid Biochem Mol Biol. 2010;122:28–34.
Laforest S, Pelletier M, Denver N, Poirier B, Nguyen S, Walker BR, et al. Simultaneous quantification of estrogens and glucocorticoids in human adipose tissue by liquid-chromatography-tandem mass spectrometry. J Steroid Biochem Mol Biol. 2019;195:105476.
Springer NL, Iyengar NM, Bareja R, Verma A, Jochelson MS, Giri DD, et al. Obesity-associated extracellular matrix remodeling promotes a macrophage phenotype similar to tumor-associated macrophages. Am J Pathol. 2019;189:2019–35.
Lackey DE, Burk DH, Ali MR, Mostaedi R, Smith WH, Park J, et al. Contributions of adipose tissue architectural and tensile properties toward defining healthy and unhealthy obesity. Am J Physiol Endocrinol Metab. 2014;306:E233–46.
Iyengar NM, Brown KA, Zhou XK, Gucalp A, Subbaramaiah K, Giri DD, et al. Metabolic obesity, adipose inflammation and elevated breast aromatase in women with normal body mass index. Cancer Prev Res. 2017;10:235–43.
Michaud A, Drolet R, Noël S, Paris G, Tchernof A. Visceral fat accumulation is an indicator of adipose tissue macrophage infiltration in women. Metabolism. 2012;61:689–98.
Sanchez-Navarro I, Gamez-Pozo A, Gonzalez-Baron M, Pinto-Marin A, Hardisson D, Lopez R, et al. Comparison of gene expression profiling by reverse transcription quantitative PCR between fresh frozen and formalin-fixed, paraffin-embedded breast cancer tissues. Biotechniques. 2010;48:389–97.
Acknowledgements
The authors thank all research team members who were involved in data acquisition and patient’s recruitment, including Lucie Tellier, Isabelle Dumas and Karine Plourde. We express gratitude to all the participants for allowing access to their biological samples and data, and for the time they devoted to answer questionnaires. This work was supported by an Intercenter Structuring Initiative of the Cardiometabolic health, Diabetes and Obesity (CMDO) Research Network to CD, AT and FD, and grants to CD from the Canadian Breast Cancer Research Alliance (#20462), the Fondation du cancer du sein du Québec and the Banque de tissus et données of the Réseau de recherche sur le cancer of the Fonds de recherche du Québec-santé (FRQS) associated with the Canadian Tumour Repository Network (CTRNet). SL is the recipient of Ph.D. scholarships from the FRQS and the Canadian Institutes of Health Research (CIHR) (GSD 154162). CD holds an Investigator Awards (Senior) from the FRQS.
Author information
Authors and Affiliations
Contributions
SL designed research study; conducted experiments; acquired, analysed and interpreted data; wrote the paper; critically revised the paper; and gave final approval. KEI provided pathologic examination of tissue specimens; contributed to acquire data and interpreted data; critically revised the paper; and gave final approval. GO conducted experiments; critically revised the paper; and gave final approval. MFG conducted experiments; contributed to acquire data; critically revised the paper; and gave final approval. AM provided biological specimens and experimental protocols; critically revised the paper; and gave final approval. FD funded the study and gave final approval. AT funded and designed the study; interpreted data; critically revised the paper; gave final approval; and supervised the study. CD funded and designed the study; acquired, analysed and interpreted data; critically revised the paper; gave final approval; and supervised the study.
Corresponding author
Ethics declarations
Conflict of interest
AT is the recipient of research grant support from Johnson & Johnson Medical Companies and Medtronic for studies unrelated to this publication. He has received consulting fees from Bausch Health. The remaining authors have no potential conflicts of interest to declare.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Laforest, S., Ennour-Idrissi, K., Ouellette, G. et al. Associations between markers of mammary adipose tissue dysfunction and breast cancer prognostic factors. Int J Obes 45, 195–205 (2021). https://doi.org/10.1038/s41366-020-00676-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41366-020-00676-3
This article is cited by
-
A novel 3D culture model for human primary mammary adipocytes to study their metabolic crosstalk with breast cancer in lean and obese conditions
Scientific Reports (2023)
-
Bodywide ecological interventions on cancer
Nature Medicine (2023)
-
Targeting adipocyte–immune cell crosstalk to control breast cancer progression
Journal of Cancer Research and Clinical Oncology (2023)
-
The pleiotropic roles of adipocyte secretome in remodeling breast cancer
Journal of Experimental & Clinical Cancer Research (2022)
-
The evolving view of thermogenic fat and its implications in cancer and metabolic diseases
Signal Transduction and Targeted Therapy (2022)