Abstract
Soluble receptors are soluble forms of receptors found in the extracellular space. They have emerged as pivotal regulators of cellular signaling and disease pathogenesis. This review emphasizes their significance in cancer as diagnostic/prognostic markers and potential therapeutic targets. We provide an overview of the mechanisms by which soluble receptors are generated along with their functions. By exploring their involvement in cancer progression, metastasis, and immune evasion, we highlight the importance of soluble receptors, particularly soluble cytokine receptors and immune checkpoints, in the tumor microenvironment. Although current research has illustrated the emerging clinical relevance of soluble receptors, their therapeutic applications remain underexplored. As the landscape of cancer treatment evolves, understanding and targeting soluble receptors might pave the way for novel strategies for cancer diagnosis, prognosis, and therapy.
Similar content being viewed by others
Introduction
Soluble receptors are unique types of cellular receptors that exist in a soluble form. Receptors generally consist of a cytoplasmic domain, a transmembrane domain, and an extracellular domain. Soluble receptors are released into the extracellular space in the form of an extracellular domain lacking a transmembrane domain or bound to extracellular vesicles1. By binding to ligands in the extracellular environment, independent of their membrane-bound counterparts, soluble receptors can enhance or disrupt cellular signaling pathways2. They can also enter the circulation and elicit local and systemic effects by regulating cellular processes in various physiological conditions3. However, abnormal levels of these receptors in the circulation have been associated with disease severity across a range of conditions, including autoimmune diseases, diabetes, infectious diseases, and cancer3,4,5,6.
In cancer research, soluble receptors have recently generated interest due to their potential as biomarkers5,7,8, given their increased levels in the bodily fluids of patients. As biomarkers, they might offer benefits in early detection of cancer, prognosis estimation, and monitoring of treatment response. Beyond their diagnostic value, emerging evidence has demonstrated that soluble receptors are involved in cancer progression, metastasis, and escape from immune surveillance9,10,11,12. Specifically, soluble forms of cytokine receptors and immune checkpoints have been identified as key modulators in cancer pathogenesis. Although their clinical relevance in cancer has become increasingly apparent, their therapeutic use remains a budding field. Given the limitations of current cancer therapies, targeting soluble receptors is expected to open promising therapeutic avenues.
This review endeavors to dissect the complexities of soluble receptors in cancer. We will elucidate key mechanisms of soluble receptors, from their generation to their roles in cancer pathogenesis, with a particular focus on soluble cytokine receptors and soluble immune checkpoints. Additionally, we will delve into their clinical significance across multiple cancer types, reflecting on current research and existing therapeutic challenges. As our comprehension of soluble receptors evolves, this review highlights their potential as diagnostic/prognostic biomarkers and therapeutic targets in cancer.
Generation of soluble receptors
Given the importance of soluble receptors in the development of various diseases, a comprehensive understanding of the mechanisms involved in their generation is essential for identifying potential therapeutic targets. Soluble receptors are known to be produced by several distinct molecular mechanisms, including (1) ectodomain shedding, (2) alternative mRNA splicing, and (3) extracellular vesicle release (Fig. 1). In this section, the generation of soluble receptors by each mechanism and clinical implications will be discussed.
a Ectodomain shedding of a membrane-bound receptor. The substrate receptor is cleaved by an ADAM protease, resulting in release of a soluble receptor into the extracellular space. The remaining C-terminal fragment is further cleaved by the γ-secretase protease complex to generate an intracellular domain fragment. b Alternative splicing of a transcript encoding a receptor, generating either a membrane-bound receptor or a soluble receptor. c Release of a membrane-bound receptor in extracellular vesicles. This figure was created with BioRender.com.
Ectodomain shedding
Ectodomain shedding is a process in which transmembrane proteins exposed on the cell surface or cellular organelles are proteolytically cleaved and released by enzymes, called “sheddases”13. The cleaved extracellular domain (ectodomain) of a membrane-bound receptor is released into the extracellular space and transported in a soluble form. Enzymes known as ADAMs (a disintegrin and metalloproteinases), which are the best-characterized sheddases, are central to this process (ectodomain shedding). Within this ADAM family, ADAM10 and ADAM17, which have similar structures, are of particular interest, especially in the context of cancer research14,15,16. They consist of a catalytic metalloproteinase domain that functions in shedding, a disintegrin domain, a cysteine-rich domain, a transmembrane domain, and a C-terminal cytoplasmic domain that is involved in activity regulation. The short C-terminal fragment (CTF) that remains at the plasma membrane as a result of receptor cleavage is further processed by the γ-secretase protease complex to release the intracellular domain (ICD) fragment (Fig. 1a). Although most ICDs are degraded, some are translocated to several cellular compartments such as the nucleus and mitochondria where they are involved in intercellular signaling17.
Ectodomain shedding is known as a general mechanism for generating soluble forms of growth factor receptors and many types of cytokine receptors18. For instance, cytokine receptors cleaved by sheddases include class I cytokine receptors (e.g., IL-2 receptor, IL-6 receptor), the tumor necrosis factor (TNF) receptor superfamily, and the IL-1 receptor /Toll-like receptor superfamily3,19,20. Recent studies have indicated that serum levels of soluble receptors generated by proteolytic cleavage are correlated with disease severity in patients4,14,21. To date, considerable research has illuminated the mechanisms and roles of soluble receptors produced through ectodomain shedding. However, the underlying mechanisms governing shedding and soluble receptor generation remain elusive.
Alternative mRNA splicing
Soluble receptors can also be generated through alternative mRNA splicing, which can remove the exon encoding transmembrane domain of the receptor. When a full-length receptor is expressed, it exists in a form bound to the cell membrane through the transmembrane domain. However, when soluble form of the receptor lacking transmembrane region is expressed, it is secreted from the cell into the extracellular space (Fig. 1b). Recent studies have revealed that many soluble cytokine receptors are generated by alternative splicing as well as ectodomain shedding19,22,23,24. TNF receptor 2 (TNFR2) can undergo alternative splicing to produce a soluble isoform that lacks exons 7 and 8, which encode transmembrane and cytoplasmic domains25. This soluble TNFR2 (sTNFR2) can be detected in human serum and its levels are elevated in patients with cancer and inflammatory diseases26,27,28. In addition to cytokine receptors, several inhibitory immune checkpoints have been shown to be released in a soluble form by alternative splicing. A soluble form of PD-1 (programmed death 1) is generated by alternative splicing of exon 3, which encodes the transmembrane domain of the PD-1 gene29. Another immune checkpoint CTLA-4 (cytotoxic T lymphocyte antigen 4) is also found in a soluble form lacking the transmembrane domain, encoded by exon 3 of CTLA-4 gene30. These soluble immune checkpoints can be detected in human serum and used as diagnostic markers in patients with various cancers5,9,31.
Extracellular vesicle release
Membrane-bound receptors are also released as components of extracellular vesicles such as microvesicles and exosomes. Although the receptor itself is not in a soluble form, it remains bound to the vesicle membrane. It is then released into the extracellular space, where it can still bind to its ligand (Fig. 1c). Some cytokine receptors such as TNF receptors (TNFR1 and TNFR2) and IL-6 receptor (IL-6R) have been detected on extracellular vesicles as full-length proteins32,33,34. These circulating vesicles can affect signaling pathways in other cell types. Additionally, it has been reported that tumor-derived exosomes can carry immunosuppressive or immunostimulatory molecules on their surface to mediate the function of immune cells in the tumor microenvironment35. ADAM proteases have also been found in extracellular vesicles such as exosomes36,37, suggesting that the ectodomain of receptors on the vesicle membrane might be cleaved and released by ADAM in these vesicles. However, shedding from extracellular vesicles remains largely unexplored.
It is noteworthy that extracellular vesicles such as exosomes can fuse with other cells38. This suggests that cells that do not normally express a particular receptor can express that receptor in its full-length upon fusion with such extracellular vesicles. It has been reported that extracellular vesicles containing full-length IL-6R can be fused with distant cells lacking IL-6R, inducing long-term intracellular signaling in target cells39. In cancers, microvesicles containing epidermal growth factor receptor variant III (EGFRvIII) are released from glioma cells and transferred to other cells lacking EGFRvIII, leading to a transformed phenotype40. Similarly, EGFR-containing exosomes can be transferred from primary gastric cancer cells to liver stromal cells and promote liver metastasis41. Therefore, extracellular vesicles with full-length receptors are critical for tumor progression.
Soluble cytokine receptors in cancer
Cytokines as messengers of the immune system can modulate immune responses by orchestrating cellular functions including cell proliferation, differentiation, and migration42. When receptors are bound by their respective cytokines, they initiate a series of intracellular signaling cascades. Numerous studies have reported that their dysregulation is closely associated with the pathogenesis of inflammatory diseases and cancer42,43,44. In the context of cancer, prolonged activated or suppressed signaling of certain cytokines can foster immune evasion in the tumor microenvironment45,46. Moreover, some cytokines and their receptors can be produced by tumor cells themselves, creating an autocrine loop that further enhances cell survival and proliferation47,48.
Cytokine receptors are found in membrane-bound form and soluble forms. Soluble forms of cytokine receptors can be released into the extracellular environment, which adds another layer of regulation. By binding freely to their respective ligands, they either enhance or reduce cytokine signaling depending on the context, thereby regulating tumor growth and the surrounding microenvironment10,11,49. Notably, levels of soluble cytokine receptors have been reported to be higher in serum of patients with various cancers than in that of healthy controls (Table 1). The next section will detail the mechanisms and clinical significance of representative soluble cytokine receptors, including the soluble forms of IL-2 receptor, IL-6 receptor, and TNF receptors.
Soluble IL-2R (sIL-2R)
Interleukin-2 (IL-2) is a cytokine critical for T cell proliferation, the generation of effector and memory CD8+ T cells, and the cytotoxic activity of natural killer (NK) cells50. The IL-2 receptor (IL-2R) comprises three subunits: IL-2Rα (CD25), IL-2β (CD122), and γ-chain (γc, CD132). Of these, the α subunit can be shed by proteases from the cell surface to form soluble IL-2 receptor (sIL-2Rα)51,52,53. In serum, sIL-2Rα can modulate the biological function of IL-2 by either diminishing or enhancing IL-2-mediated effects, depending on the context. It has been reported that sIL-2Rα can compete with membrane-bound IL-2R for IL-2 binding and inhibit IL-2-mediated proliferation of memory phenotype CD8+ T cells in vitro54. In contrast, sIL-2Rα can facilitate IL-2-mediated STAT5 activation and induce Foxp3 expression in CD4+ T cells, which is critical for the generation and maintenance of regulatory T (Treg) cells, suppressing CD8+ T cell proliferation55.
Previous studies have reported that levels of sIL-2Rα are increased in patients with many cancers, including carcinoma and lymphoma56,57,58. Elevated sIL-2Rα level is correlated with high grade tumors and poor overall survival56,59, suggesting that it can be used as a non-invasive marker for the diagnosis and prognosis of cancer. Given that sIL-2R regulates immune responses, understanding its role in the tumor microenvironment can pave the way for novel anti-tumor therapies. Therefore, its clinical significance as a biomarker and a potential therapeutic target for cancer warrants further investigation.
Soluble IL-6R (sIL-6R)
The interleukin-6 (IL-6) signaling pathway is critical for various physiological processes, including inflammation, hematopoiesis, metabolism, and cancer60. The IL-6 receptor (IL-6R) exists in two forms: membrane-bound IL-6R and its soluble counterpart. In IL-6 classic signaling, IL-6 binds to membrane-bound IL-6R, inducing homodimerization of signal transducer protein gp130 (CD130) and activation of intracellular signaling cascades61,62. Soluble IL-6R (sIL-6R) can be generated by ectodomain shedding, alternative splicing, and release on extracellular vesicles19. sIL-6R retains its ability to bind to IL-6, forming the IL-6/sIL-6R complex. This complex then associate with membrane-bound gp130 homodimers, leading to intracellular signaling. This process is called IL-6 trans-signaling63. Notably, while IL-6 classic signaling through membrane-bound IL-6R is restricted to specific cell types such as hepatocytes and some lymphoid cells, IL-6 trans-signaling via sIL-6R can occur in all cells. It has been reported that gp130 is ubiquitously expressed in almost all cells except granulocytes19.
It is known that the IL-6-induced JAK/STAT3 signaling pathway drives the proliferation and survival of tumor cells62. Indeed, IL-6 trans-signaling has been reported to promote the development of pancreatic cancer and KRAS-driven lung adenocarcinoma10,64. In colitis-associated cancer (CAC), IL-6 and sIL-6R are produced by lamina propria myeloid cells. They stimulate the proliferation of premalignant intestinal epithelial cells, affecting early tumor formation65,66. During the late stages of CAC development, tumor-derived sIL-6R rather than membrane-bound IL-6R induces STAT3 activation and accelerates tumor growth67,68. In addition to signal transduction in tumor cells, IL-6 trans-signaling in immune cells affects tumor progression. It has been revealed that IL-6 trans-signaling via sIL-6R derived by myeloid cells attenuates CD4+ T helper type 1 (Th1) cell differentiation in tumor-bearing mice, leading to defective anti-tumor responses69. Moreover, IL-6 trans-signaling promotes immunosuppressive function of myeloid-derived suppressor cells (MDSCs) in breast cancer70. Given the role and significance of IL-6/sIL-6R trans-signaling in tumor progression, targeting this trans-signaling has therapeutic potential in many types of cancer.
Soluble TNFR (sTNFR)
Tumor necrosis factor (TNF) is a multifunctional cytokine that plays a role in homeostasis and disease pathogenesis71. TNF binds to two distinct receptors: TNF receptor 1 (TNFR1) and TNF receptor 2 (TNFR2). TNFR1 and TNFR2 have similar extracellular structures and are activated by both soluble and transmembrane TNF26. TNFR1 has an intracellular death domain and induces inflammation and tissue degeneration as well as programmed cell death. In contrast, TNFR2 lacks a death domain and mediates primarily homeostatic effects, including cell survival, proliferation, and tissue regeneration71. Both TNFR1 and TNFR2 can exist in soluble and membrane-bound forms. Soluble TNFRs are generated by proteolytic cleavage, synthesis via alternative mRNA splicing, or release in extracellular vesicles3. TNFRs are cleaved by ADAM17, also known as TACE (TNF-α converting enzyme)72. It has been shown that levels of soluble TNFR1 (sTNFR1) and soluble TNFR2 (sTNFR2) are increased in several diseases, including type 1 and type 2 diabetes with chronic kidney diseases73,74.
Previous studies have shown that the TNFR2-expressing Treg subset has a highly immunosuppressive function75,76. Additionally, TNFR2 has been reported to be expressed on CD8+ regulatory T cells (CD8+ Tregs) and CD8+ effector T cells, thus coordinating immune responses77. It is noteworthy that TNFR2 is increased in tumor-infiltrating Treg cells from human solid tumors78. In murine lung cancer and melanoma models, tumor growth in TNFR2-deficient mice was significantly decreased compared to that in wild-type mice79. Levels of sTNFR2 in serum/plasma samples of several cancer patients are elevated, and such elevation has been found to be correlated with cancer development and poor overall survival80. These findings suggest that circulating sTNFR2 plays a pivotal role in the tumor microenvironment and can be used as a biomarker for cancer diagnosis and prognosis as well as cancer therapy.
Soluble immune checkpoints in cancer
Immune checkpoints are paired receptor-ligand molecules that fine-tune the immune system to maintain immune homeostasis. Recently, immune checkpoints have gained attention in cancer immunotherapy, due to their exploitation by tumor cells rather than their protective role81,82. Overall, discovery of their roles in tumor immune evasion has paved the way for immune checkpoint blockade as a revolutionary therapeutic approach. In recent decades, antibodies targeting immune checkpoints, such as CTLA-4 and PD-1, have been actively developed and studied for cancer treatment82. However, clinical trials of immune checkpoint blockade to date have revealed limitations, as the percentage of patients who respond to such treatment is still low83. For example, although combination therapy with anti-PD-1 antibody nivolumab and anti-CTLA-4 antibody ipilimumab has been demonstrated to have therapeutic effects on overall survival outcomes in patients with advanced melanoma84,85, only a few patients can benefit from this treatment. Due to such limitations in the development of immunotherapies for cancer, an in-depth understanding of the mechanism of immune checkpoints has become necessary.
In addition to being expressed on cell membranes, immune checkpoints can be found in soluble form. Several studies have identified the source of specific soluble immune checkpoints. For example, soluble form of programmed cell death ligand 1 (sPD-L1) is reported to be produced by tumor cells or activated mature dendritic cells86,87. sPD-L1 can be generated via ectodomain shedding and binds to PD-1 to inhibit T cell responses87,88. Moreover, it has been reported that soluble CTLA-4 (sCTLA-4) is produced by Treg cells through alternative mRNA splicing89, and the spliced variant has also been detected in monocytes and immature dendritic cells90. Although the major sources of several soluble immune checkpoints have been identified in vitro, the molecular mechanisms responsible for the generation and physiological function of soluble immune checkpoints in vivo still require further investigation.
Recent studies have shown that soluble forms of immune checkpoints can be detected in human serum or plasma and that elevated levels are associated with many cancer types7,9,91,92. Levels of these soluble immune checkpoints are not only simply increased in cancer patients but also correlated with the disease severity and prognosis of patients (Table 2). Moreover, high serum levels of soluble immune checkpoints are associated with resistance to several targeted cancer therapies in cancer patients93,94,95. These findings suggest the potential of using circulating immune checkpoints as biomarkers for the diagnosis and prognosis of various cancers.
Targeting soluble receptors for cancer therapy
Therapeutics that directly target soluble receptors
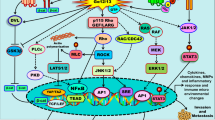
Soluble receptors not only have the potential to be used as biomarkers for cancer diagnosis and prognosis, but can also be used as therapeutic targets in cancer treatment (Fig. 2). Direct targeting of soluble receptors or their pathways might be an effective therapeutic strategy to enhance anti-tumor responses. Although additional research is still needed, the clinical significance of increased soluble receptor levels in patients with various types of cancer provides sufficient evidence to support the development of novel cancer treatments by targeting these soluble receptors. Circulating levels of sIL-2Rα, one of the soluble cytokine receptors, have been shown to be correlated with progression of several types of cancer in previous studies96,97,98, with potential for use as a diagnostic and prognostic marker for cancer. However, considering that it is highly correlated not only with cancer but also with other diseases such as inflammatory diseases3, it can be used as an indicator to confirm the activation of T cells that produce sIL-2Rα in pathogenic conditions rather than simply as an indicator of cancer49. Therefore, when directly targeting soluble receptors as a cancer treatment strategy, it is important to conduct a precise analysis according to the characteristics of each cancer type and understand the molecular mechanism of each soluble receptor. To date, small-molecule compounds or monoclonal antibodies that directly target several soluble receptors have been developed, and research into the mechanism of how these substances affect the tumor microenvironment and their efficacy in both preclinical and clinical trials is ongoing.
Targeting soluble receptors has benefits in cancer diagnosis, prognosis, and treatment. Considering that high levels of soluble receptors are detected in the bodily fluids of cancer patients and that such high levels are associated with disease severity, soluble receptors have the potential to be used as minimally invasive biomarkers for early detection and prognosis of cancer. Additionally, blocking soluble receptors through various therapeutic strategies can potentially improve the efficacy of current cancer treatment. This figure was created with BioRender.com.
One of the therapeutic strategies being actively investigated is blocking IL-6 trans-signaling99,100. Some humanized monoclonal antibodies targeting IL-6R, such as tocilizumab and sarilumab, have been developed to inhibit IL-6 signaling. They are currently approved for treating arthritis or are in clinical trials for other diseases101,102,103. However, the problem with these antibodies is that they cannot distinguish between membrane-bound and soluble forms of IL-6R, inhibiting IL-6 classic signaling and trans-signaling at the same time. An alternative IL-6 trans-signaling inhibitor that can be considered is a soluble gp130–Fc fusion protein (sgp130Fc, also known as olamkicept), which is a fusion protein of the extracellular portion of gp130 and the Fc region of a human IgG1 antibody104. IL-6 classic signaling maintains local homeostasis even under normal healthy conditions; under inflammatory conditions, however, local IL-6 classic signaling and trans-signaling as well as systemic trans-signaling are induced by high levels of IL-6 and sIL-6R in the blood100. Therefore, sgp130Fc, which selectively inhibits only trans-signaling without affecting IL-6 classic signaling, has high potential as a therapeutic agent for various diseases. As mentioned above, IL-6 trans-signaling in various cancer types promotes tumor progression by suppressing anti-tumor responses of immune cells as well as increasing cell growth through signal transduction in tumor cells themselves. Surprisingly, blocking IL-6 trans-signaling with sgp130Fc has therapeutic effects on reducing tumor progression in murine cancer models, including murine colitis-associated cancer (CAC)65,66, lung adenocarcinoma10, and hepatocellular carcinoma (HCC) models105,106. However, few studies on blockade of IL-6 trans-signaling in cancer patients have been reported, despite its therapeutic effects in numerous preclinical cancer models. Therefore, additional clinical studies are needed before it can be used as a clinical tool for cancer treatment.
Another strategy to directly target soluble receptors for cancer treatment is to inhibit sTNFR. TNFR2-expressing Tregs are increased in the tumor microenvironment and have a high suppressive capacity in various cancers, including ovarian cancer, acute myeloid leukemia, and lung cancer107,108,109. It has been revealed that newly identified TNFR2 antagonistic monoclonal antibodies (TNFR2 antagonists) inhibit soluble TNFR2 secretion and Treg proliferation in vitro110. Of note, a TNFR2 antagonist has a greater effect on suppressing Tregs from the ascites fluid of ovarian cancer patients than Tregs from the peripheral blood of healthy donors110, suggesting the specificity of TNFR2 antagonists for the tumor microenvironment. Specifically, inhibiting the activity of tumor-residing Tregs through TNFR2 antagonism can increase proliferation of effector T cells in the tumor microenvironment and suppress tumor growth. Thus, it can be considered an engaging cancer therapy.
Modulation of ectodomain shedding
Inhibiting enzymes responsible for shedding of membrane-bound receptors can reduce levels of soluble receptors, which might also be a therapeutic strategy for cancer. Recent studies have shown that expression levels of ADAMs are increased in multiple cancer types111,112. Preclinical studies have reported that small-molecule compounds or monoclonal antibodies for modulating ADAMs inhibit migration, invasion, and growth of tumor cells112. For instance, treatment with Aderbasib (INCB7839), a small-molecule inhibitor of ADAM10/ADAM17, was reported to prevent growth of HER2+ human breast cancer in a mouse xenograft model113. INCB7839 has also been tested in clinical trials, with promising results in phase I/II trials of Trastuzumab-based HER2+ breast cancer therapy by inhibiting HER2 shedding (NCT01254136), and evaluated in phase I trials for recurrent or progressive high-grade gliomas (NCT04295759).
Targeting catalytic domains (metalloprotease domains) of the ADAM protease has so far failed due to highly conserved active sites among ADAM enzymes, resulting in unfavorable toxic effects. Interestingly, recent studies have revealed that non-catalytic domains of ADAM10 and ADAM17, specifically a disintegrin domain and a cysteine-rich domain, can provide substrate specificity114. Through additional research, it is expected that inhibitors with increased specificity for other ADAM families with different structures will be developed, which will provide a way to overcome the limitations in ADAM inhibitor development. There are several ongoing clinical trials targeting specific ADAM proteases for cancer. The ADAM9-targeting antibody-drug conjugate IMGC936 has been tested in phase I/II trials for advanced solid tumors, such as non-squamous non-small cell lung cancer, triple-negative breast cancer, and colorectal cancer (NCT04622774). Therefore, targeting specific ADAM proteases may be a promising therapeutic strategy for cancer.
Enhancers of existing therapies: combination therapy
Understanding the interplay between soluble receptors and established therapeutic strategies can lead to more effective treatments. Despite the clinical success of current cancer immunotherapies, such as immune checkpoint blockade, a significant proportion of cancer patients still do not respond to treatment or are resistant to inhibitor treatment115. Of note, serum levels of soluble immune checkpoints are correlated with resistance to immunotherapy. In patients with advanced melanoma, anti-PD-1 antibody (pembrolizumab) monotherapy has been reported to increase serum levels of lymphocyte-activation gene 3 (sLAG3) in a disease progression group compared to a control group95. Additionally, serum PD-1 levels are increased in melanoma patients with disease progression, following combination treatment with anti-PD-1 antibody (nivolumab) plus anti-CTLA-4 antibody (ipilimumab)95. These recent findings suggest that targeting soluble immune checkpoints might be beneficial for immunotherapy-resistant cancer patients. Therefore, combination therapy with existing therapeutic strategies and inhibition of soluble receptors might be a solution to overcome the limitations of current treatments.
Challenges and future directions
Therapeutic strategies for cancer by inhibiting IL-6 trans-signaling should consider the effects of other IL-6 family members. The IL-6 family consists of IL-6, IL-11, and IL-27, all of which transduce signals using the gp130 receptor20. Of note, the IL-11 receptor (IL-11R) can also be detected in a soluble form. Levels of soluble IL-11R have been reported to be elevated in patients with gastric cancer116, suggesting possible effects of IL-11 trans-signaling in cancer progression in vivo. To reduce the potential of side effects, second-generation and third-generation variants have been developed from the previously developed sgp130Fc100. Indeed, the selectivity of inhibitors for IL-6 trans-signaling has gradually increased from the first-generation sgp130Fc to the second-generation variant sgp130FLYRFc and the third-generation variant cs130Fly, but the effect on IL-11 trans-signaling has gradually decreased117,118. Therefore, increasing the selectivity of inhibitors targeting specific soluble receptor signaling will be of great help in reducing unwanted side effects in clinical studies.
In addition, several sheddase inhibitors are currently being developed for cancer treatment. While the specificity between ADAM family members has been addressed to some extent in the development of ADAM-targeted inhibitors, the fact that each ADAM can cleave a variety of substrates, including multiple cytokines, growth factors, and other membrane-bound receptors, may lead to detrimental side effects in clinical studies18. A recent study revealed that MEDI3622, a specific ADAM17 inhibitory antibody, has the potential to inhibit not only the HER pathway but also the EGFR pathway119. The researchers used combination therapy with MEDI3622 and the EGFR inhibitor cetuximab and found synergic effects resulting in complete tumor regression in an OE21 esophageal xenograft model119. Therefore, in future studies of sheddase inhibitors, it is necessary to first analyze expression of multiple substrates in each patient with a specific cancer type.
Conclusions
Soluble receptors have evolved as crucial players in cancer research. Their generation through various mechanisms, such as ectodomain shedding, alternative mRNA splicing, and extracellular vesicle release, underscores multifaceted ways in which they regulate cellular signaling pathways. Focusing on the roles of soluble cytokine receptors and soluble immune checkpoints, this review highlights the indispensable role of soluble receptors in cancer progression, metastasis, and immune evasion. Soluble receptors are detected at high levels in the blood of patients with various cancers. Given that soluble receptors are present in bodily fluids, they might provide a minimally invasive method for early diagnosis and prognosis of cancer. In addition to early cancer detection, directly targeting soluble receptors using small-molecule compounds or monoclonal antibodies could be considered as cancer treatment strategies. The therapeutic potential of targeting these soluble receptors offers promising avenues for cancer treatment, and strategically combining therapies may enhance the efficacy of current strategies. Nevertheless, in the case of soluble immune checkpoints, how direct regulation of soluble immune checkpoints affects the tumor microenvironment has not yet been elucidated. Hence, it is evident that more studies are needed, especially in harnessing soluble receptors for cancer treatment. Future endeavors in this area should focus on improving therapeutic strategies, addressing identified challenges, and understanding the long-term implications of targeting soluble receptors in cancer.
References
Heaney, M. L. & Golde, D. W. Soluble receptors in human disease. J. Leukoc. Biol. 64, 135–146 (1998).
Levine, S. J. Mechanisms of soluble cytokine receptor generation. J. Immunol. 173, 5343–5348 (2004).
Kefaloyianni, E. Soluble forms of cytokine and growth factor receptors: mechanisms of generation and modes of action in the regulation of local and systemic inflammation. FEBS Lett. 596, 589–606 (2022).
Li, W. et al. Soluble immune checkpoints are dysregulated in COVID-19 and heavy alcohol users with HIV infection. Front. Immunol. 13, 833310 (2022).
Chakrabarti, R., Kapse, B. & Mukherjee, G. Soluble immune checkpoint molecules: serum markers for cancer diagnosis and prognosis. Cancer Rep. Hoboken 2, e1160 (2019).
Gohda, T. et al. Circulating TNF receptors 1 and 2 predict stage 3 CKD in type 1 diabetes. J. Am. Soc. Nephrol. 23, 516–524 (2012).
Swiderska, J. et al. Clinical relevance of soluble forms of immune checkpoint molecules sPD-1, sPD-L1, and sCTLA-4 in the diagnosis and prognosis of ovarian cancer. Diagnostics (Basel) 12, 189 (2022).
Reichl, P. & Mikulits, W. Accuracy of novel diagnostic biomarkers for hepatocellular carcinoma: an update for clinicians (Review). Oncol. Rep. 36, 613–625 (2016).
Wang, Q. et al. Soluble immune checkpoint-related proteins as predictors of tumor recurrence, survival, and T cell phenotypes in clear cell renal cell carcinoma patients. J. Immunother. Cancer 7, 334 (2019).
Brooks, G. D. et al. IL6 trans-signaling promotes KRAS-driven lung carcinogenesis. Cancer Res. 76, 866–876 (2016).
Badoual, C. et al. The soluble alpha chain of interleukin-15 receptor: a proinflammatory molecule associated with tumor progression in head and neck cancer. Cancer Res. 68, 3907–3914 (2008).
Gu, D., Ao, X., Yang, Y., Chen, Z. & Xu, X. Soluble immune checkpoints in cancer: production, function and biological significance. J. Immunother. Cancer 6, 132 (2018).
Lichtenthaler, S. F., Lemberg, M. K. & Fluhrer, R. Proteolytic ectodomain shedding of membrane proteins in mammals-hardware, concepts, and recent developments. EMBO J. 37, e99456 (2018).
Miller, M. A., Sullivan, R. J. & Lauffenburger, D. A. Molecular pathways: receptor ectodomain shedding in treatment, resistance, and monitoring of cancer. Clin. Cancer Res. 23, 623–629 (2017).
Smith, T. M. Jr., Tharakan, A. & Martin, R. K. Targeting ADAM10 in cancer and autoimmunity. Front. Immunol. 11, 499 (2020).
Dusterhoft, S., Lokau, J. & Garbers, C. The metalloprotease ADAM17 in inflammation and cancer. Pathol. Res. Pr. 215, 152410 (2019).
Merilahti, J. A. M. & Elenius, K. Gamma-secretase-dependent signaling of receptor tyrosine kinases. Oncogene 38, 151–163 (2019).
Huovila, A. P., Turner, A. J., Pelto-Huikko, M., Karkkainen, I. & Ortiz, R. M. Shedding light on ADAM metalloproteinases. Trends Biochem. Sci. 30, 413–422 (2005).
Schumertl, T., Lokau, J., Rose-John, S. & Garbers, C. Function and proteolytic generation of the soluble interleukin-6 receptor in health and disease. Biochim Biophys. Acta. Mol. Cell Res. 1869, 119143 (2022).
Lokau, J. & Garbers, C. Biological functions and therapeutic opportunities of soluble cytokine receptors. Cytokine Growth Factor Rev. 55, 94–108 (2020).
Arai, J., Otoyama, Y., Nozawa, H., Kato, N. & Yoshida, H. The immunological role of ADAMs in the field of gastroenterological chronic inflammatory diseases and cancers: a review. Oncogene 42, 549–558 (2023).
Bulanova, E. et al. Retraction: Soluble interleukin (IL)-15Ralpha is generated by alternative splicing or proteolytic cleavage and forms functional complexes with IL-15. J. Biol. Chem. 286, 5934 (2011).
Blum, H., Wolf, M., Enssle, K., Rollinghoff, M. & Gessner, A. Two distinct stimulus-dependent pathways lead to production of soluble murine interleukin-4 receptor. J. Immunol. 157, 1846–1853 (1996).
Lust, J. A. et al. Isolation of an mRNA encoding a soluble form of the human interleukin-6 receptor. Cytokine 4, 96–100 (1992).
Lainez, B. et al. Identification and characterization of a novel spliced variant that encodes human soluble tumor necrosis factor receptor 2. Int. Immunol. 16, 169–177 (2004).
Josephs, S. F. et al. Unleashing endogenous TNF-alpha as a cancer immunotherapeutic. J. Transl. Med. 16, 242 (2018).
Aderka, D. et al. Increased serum levels of soluble receptors for tumor necrosis factor in cancer patients. Cancer Res. 51, 5602–5607 (1991).
Diez-Ruiz, A. et al. Soluble receptors for tumour necrosis factor in clinical laboratory diagnosis. Eur. J. Haematol. 54, 1–8 (1995).
Nielsen, C., Ohm-Laursen, L., Barington, T., Husby, S. & Lillevang, S. T. Alternative splice variants of the human PD-1 gene. Cell Immunol. 235, 109–116 (2005).
Oaks, M. K. et al. A native soluble form of CTLA-4. Cell Immunol. 201, 144–153 (2000).
Zhu, X. & Lang, J. Soluble PD-1 and PD-L1: predictive and prognostic significance in cancer. Oncotarget 8, 97671–97682 (2017).
Hawari, F. I. et al. Release of full-length 55-kDa TNF receptor 1 in exosome-like vesicles: a mechanism for generation of soluble cytokine receptors. Proc. Natl Acad. Sci. USA 101, 1297–1302 (2004).
Soderberg, A., Barral, A. M., Soderstrom, M., Sander, B. & Rosen, A. Redox-signaling transmitted in trans to neighboring cells by melanoma-derived TNF-containing exosomes. Free Radic. Biol. Med. 43, 90–99 (2007).
Schumacher, N. et al. Shedding of endogenous Interleukin-6 Receptor (IL-6R) is governed by A Disintegrin and Metalloproteinase (ADAM) proteases while a full-length IL-6R isoform localizes to circulating microvesicles. J. Biol. Chem. 290, 26059–26071 (2015).
Whiteside, T. L. Tumor-derived exosomes and their role in cancer progression. Adv. Clin. Chem. 74, 103–141 (2016).
Keller, M. D. et al. Decoy exosomes provide protection against bacterial toxins. Nature 579, 260–264 (2020).
Shimoda, M. & Khokha, R. Metalloproteinases in extracellular vesicles. Biochim. Biophys. Acta. Mol. Cell Res. 1864, 1989–2000 (2017).
Pegtel, D. M. & Gould, S. J. Exosomes. Annu. Rev. Biochem 88, 487–514 (2019).
Arnold, P. et al. Joint reconstituted signaling of the IL-6 receptor via extracellular vesicles. Cells 9, 1307 (2020).
Al-Nedawi, K. et al. Intercellular transfer of the oncogenic receptor EGFRvIII by microvesicles derived from tumour cells. Nat. Cell Biol. 10, 619–624 (2008).
Zhang, H. et al. Exosome-delivered EGFR regulates liver microenvironment to promote gastric cancer liver metastasis. Nat. Commun. 8, 15016 (2017).
Kany, S., Vollrath, J. T. & Relja, B. Cytokines in inflammatory disease. Int. J. Mol. Sci. 20, 6008 (2019).
Akdis, M. et al. Interleukins (from IL-1 to IL-38), interferons, transforming growth factor beta, and TNF-alpha: Receptors, functions, and roles in diseases. J. Allergy Clin. Immunol. 138, 984–1010 (2016).
Slifka, M. K. & Whitton, J. L. Clinical implications of dysregulated cytokine production. J. Mol. Med. Berl. 78, 74–80 (2000).
Labani-Motlagh, A., Ashja-Mahdavi, M. & Loskog, A. The tumor microenvironment: a milieu hindering and obstructing antitumor immune responses. Front. Immunol. 11, 940 (2020).
Briukhovetska, D. et al. Interleukins in cancer: from biology to therapy. Nat. Rev. Cancer 21, 481–499 (2021).
Haga, A., Funasaka, T., Niinaka, Y., Raz, A. & Nagase, H. Autocrine motility factor signaling induces tumor apoptotic resistance by regulations Apaf-1 and Caspase-9 apoptosome expression. Int J. Cancer 107, 707–714 (2003).
Kartikasari, A. E. R., Huertas, C. S., Mitchell, A. & Plebanski, M. Tumor-induced inflammatory cytokines and the emerging diagnostic devices for cancer detection and prognosis. Front. Oncol. 11, 692142 (2021).
Bien, E. & Balcerska, A. Serum soluble interleukin 2 receptor alpha in human cancer of adults and children: a review. Biomarkers 13, 1–26 (2008).
Liao, W., Lin, J. X. & Leonard, W. J. Interleukin-2 at the crossroads of effector responses, tolerance, and immunotherapy. Immunity 38, 13–25 (2013).
Sheu, B. C. et al. A novel role of metalloproteinase in cancer-mediated immunosuppression. Cancer Res. 61, 237–242 (2001).
El Houda Agueznay, N. et al. Soluble interleukin-2 receptor and metalloproteinase-9 expression in head and neck cancer: prognostic value and analysis of their relationships. Clin. Exp. Immunol. 150, 114–123 (2007).
Rubin, L. A., Galli, F., Greene, W. C., Nelson, D. L. & Jay, G. The molecular basis for the generation of the human soluble interleukin 2 receptor. Cytokine 2, 330–336 (1990).
Rubinstein, M. P. et al. Converting IL-15 to a superagonist by binding to soluble IL-15Ralpha. Proc. Natl Acad. Sci. USA 103, 9166–9171 (2006).
Yang, Z. Z. et al. Soluble IL-2Ralpha facilitates IL-2-mediated immune responses and predicts reduced survival in follicular B-cell non-Hodgkin lymphoma. Blood 118, 2809–2820 (2011).
Tartour, E. et al. Serum soluble interleukin-2 receptor concentrations as an independent prognostic marker in head and neck cancer. Lancet 357, 1263–1264 (2001).
Yoshida, N. et al. Clinical significance of sIL-2R levels in B-cell lymphomas. PLoS One 8, e78730 (2013).
Izzo, F. et al. Soluble interleukin-2 receptor levels in hepatocellular cancer: a more sensitive marker than alfa fetoprotein. Ann. Surg. Oncol. 6, 178–185 (1999).
Ottaiano, A. et al. Soluble interleukin-2 receptor in stage I-III melanoma. Cytokine 33, 150–155 (2006).
Hunter, C. A. & Jones, S. A. IL-6 as a keystone cytokine in health and disease. Nat. Immunol. 16, 448–457 (2015).
Skiniotis, G., Boulanger, M. J., Garcia, K. C. & Walz, T. Signaling conformations of the tall cytokine receptor gp130 when in complex with IL-6 and IL-6 receptor. Nat. Struct. Mol. Biol. 12, 545–551 (2005).
Johnson, D. E., O’Keefe, R. A. & Grandis, J. R. Targeting the IL-6/JAK/STAT3 signalling axis in cancer. Nat. Rev. Clin. Oncol. 15, 234–248 (2018).
Chalaris, A., Garbers, C., Rabe, B., Rose-John, S. & Scheller, J. The soluble Interleukin 6 receptor: generation and role in inflammation and cancer. Eur. J. Cell Biol. 90, 484–494 (2011).
Lesina, M. et al. Stat3/Socs3 activation by IL-6 transsignaling promotes progression of pancreatic intraepithelial neoplasia and development of pancreatic cancer. Cancer Cell 19, 456–469 (2011).
Grivennikov, S. et al. IL-6 and Stat3 are required for survival of intestinal epithelial cells and development of colitis-associated cancer. Cancer Cell 15, 103–113 (2009).
Matsumoto, S. et al. Essential roles of IL-6 trans-signaling in colonic epithelial cells, induced by the IL-6/soluble-IL-6 receptor derived from lamina propria macrophages, on the development of colitis-associated premalignant cancer in a murine model. J. Immunol. 184, 1543–1551 (2010).
Becker, C. et al. TGF-beta suppresses tumor progression in colon cancer by inhibition of IL-6 trans-signaling. Immunity 21, 491–501 (2004).
Becker, C. et al. IL-6 signaling promotes tumor growth in colorectal cancer. Cell Cycle 4, 217–220 (2005).
Tsukamoto, H. et al. Soluble IL6R expressed by myeloid cells reduces tumor-specific Th1 differentiation and drives tumor progression. Cancer Res. 77, 2279–2291 (2017).
Jiang, M. et al. Interleukin-6 trans-signaling pathway promotes immunosuppressive myeloid-derived suppressor cells via suppression of suppressor of cytokine signaling 3 in breast cancer. Front. Immunol. 8, 1840 (2017).
Kalliolias, G. D. & Ivashkiv, L. B. TNF biology, pathogenic mechanisms and emerging therapeutic strategies. Nat. Rev. Rheumatol. 12, 49–62 (2016).
Dri, P. et al. TNF-Induced shedding of TNF receptors in human polymorphonuclear leukocytes: role of the 55-kDa TNF receptor and involvement of a membrane-bound and non-matrix metalloproteinase. J. Immunol. 165, 2165–2172 (2000).
Niewczas, M. A. et al. Circulating TNF receptors 1 and 2 predict ESRD in type 2 diabetes. J. Am. Soc. Nephrol. 23, 507–515 (2012).
Carlsson, A. C. et al. Association of soluble tumor necrosis factor receptors 1 and 2 with nephropathy, cardiovascular events, and total mortality in type 2 diabetes. Cardiovasc. Diabetol. 15, 40 (2016).
Chen, X. et al. Co-expression of TNFR2 and CD25 identifies more of the functional CD4+FOXP3+ regulatory T cells in human peripheral blood. Eur. J. Immunol. 40, 1099–1106 (2010).
Chen, X. et al. Cutting edge: expression of TNFR2 defines a maximally suppressive subset of mouse CD4+CD25+FoxP3+ T regulatory cells: applicability to tumor-infiltrating T regulatory cells. J. Immunol. 180, 6467–6471 (2008).
Ye, L. L., Wei, X. S., Zhang, M., Niu, Y. R. & Zhou, Q. The significance of tumor necrosis factor receptor type II in CD8(+) regulatory T cells and CD8(+) effector T cells. Front. Immunol. 9, 583 (2018).
Williams, G. S. et al. Phenotypic screening reveals TNFR2 as a promising target for cancer immunotherapy. Oncotarget 7, 68278–68291 (2016).
Sasi, S. P. et al. Breaking the ‘harmony’ of TNF-alpha signaling for cancer treatment. Oncogene 31, 4117–4127 (2012).
Kartikasari, A. E. R. et al. Elevation of circulating TNF receptor 2 in cancer: a systematic meta-analysis for its potential as a diagnostic cancer biomarker. Front. Immunol. 13, 918254 (2022).
Sharma, P. & Allison, J. P. The future of immune checkpoint therapy. Science 348, 56–61 (2015).
Korman, A. J., Garrett-Thomson, S. C. & Lonberg, N. The foundations of immune checkpoint blockade and the ipilimumab approval decennial. Nat. Rev. Drug Discov. 21, 509–528 (2022).
Osipov, A. et al. Tumor mutational burden, toxicity, and response of immune checkpoint inhibitors targeting PD(L)1, CTLA-4, and combination: a meta-regression analysis. Clin. Cancer Res. 26, 4842–4851 (2020).
Hodi, F. S. et al. Combined nivolumab and ipilimumab versus ipilimumab alone in patients with advanced melanoma: 2-year overall survival outcomes in a multicentre, randomised, controlled, phase 2 trial. Lancet Oncol. 17, 1558–1568 (2016).
Larkin, J. et al. Five-year survival with combined Nivolumab and Ipilimumab in advanced melanoma. N. Engl. J. Med. 381, 1535–1546 (2019).
Frigola, X. et al. Identification of a soluble form of B7-H1 that retains immunosuppressive activity and is associated with aggressive renal cell carcinoma. Clin. Cancer Res. 17, 1915–1923 (2011).
Frigola, X. et al. Soluble B7-H1: differences in production between dendritic cells and T cells. Immunol. Lett. 142, 78–82 (2012).
Chen, Y. et al. Development of a sandwich ELISA for evaluating soluble PD-L1 (CD274) in human sera of different ages as well as supernatants of PD-L1+ cell lines. Cytokine 56, 231–238 (2011).
Ward, F. J. et al. The soluble isoform of CTLA-4 as a regulator of T-cell responses. Eur. J. Immunol. 43, 1274–1285 (2013).
Laurent, S. et al. CTLA-4 is expressed by human monocyte-derived dendritic cells and regulates their functions. Hum. Immunol. 71, 934–941 (2010).
Malinga, N. Z. et al. Systemic levels of the soluble co-inhibitory immune checkpoints, CTLA-4, LAG-3, PD-1/PD-L1 and TIM-3 are markedly increased in basal cell carcinoma. Transl. Oncol. 19, 101384 (2022).
Wang, Q. et al. Soluble immune checkpoint-related proteins in blood are associated with invasion and progression in non-small cell lung cancer. Front. Immunol. 13, 887916 (2022).
Leung, A. M. et al. Clinical benefit from Ipilimumab therapy in melanoma patients may be associated with serum CTLA4 levels. Front Oncol. 4, 110 (2014).
Dong, M. P. et al. Clinical significance of circulating soluble immune checkpoint proteins in sorafenib-treated patients with advanced hepatocellular carcinoma. Sci. Rep. 10, 3392 (2020).
Machiraju, D. et al. Soluble immune checkpoints and T-cell subsets in blood as biomarkers for resistance to immunotherapy in melanoma patients. Oncoimmunology 10, 1926762 (2021).
Nakata, B. et al. Serum soluble interleukin-2 receptor level as a prognostic indicator in gastric cancer. Br. J. Cancer 77, 1820–1824 (1998).
Cabrera, R. et al. Serum levels of soluble CD25 as a marker for hepatocellular carcinoma. Oncol. Lett. 4, 840–846 (2012).
Hashimoto, Y. et al. Prognostic importance of the soluble form of IL-2 receptoralpha (sIL-2Ralpha) and its relationship with surface expression of IL-2Ralpha (CD25) of lymphoma cells in diffuse large B-cell lymphoma treated with CHOP-like regimen with or without rituximab: a retrospective analysis of 338 cases. J. Clin. Exp. Hematop. 53, 197–205 (2013).
Rose-John, S. Blocking only the bad side of IL-6 in inflammation and cancer. Cytokine 148, 155690 (2021).
John, R. S., Jenkins, B. J., Garbers, C., Moll, J. M. & Scheller, J. Targeting IL-6 trans-signalling: past. Nat. Rev. Immunol. 23, 666–681 (2023).
Garbers, C., Heink, S., Korn, T. & Rose-John, S. Interleukin-6: designing specific therapeutics for a complex cytokine. Nat. Rev. Drug Discov. 17, 395–412 (2018).
Group, R. C. Tocilizumab in patients admitted to hospital with COVID-19 (RECOVERY): a randomised, controlled, open-label, platform trial. Lancet 397, 1637–1645 (2021).
Boyce, E. G., Rogan, E. L., Vyas, D., Prasad, N. & Mai, Y. Sarilumab: review of a second IL-6 receptor antagonist indicated for the treatment of rheumatoid arthritis. Ann. Pharmacother. 52, 780–791 (2018).
Jostock, T. et al. Soluble gp130 is the natural inhibitor of soluble interleukin-6 receptor transsignaling responses. Eur. J. Biochem 268, 160–167 (2001).
Bergmann, J. et al. IL-6 trans-signaling is essential for the development of hepatocellular carcinoma in mice. Hepatology 65, 89–103 (2017).
Rosenberg, N. et al. Combined hepatocellular-cholangiocarcinoma derives from liver progenitor cells and depends on senescence and IL-6 trans-signaling. J. Hepatol. 77, 1631–1641 (2022).
Govindaraj, C. et al. Impaired Th1 immunity in ovarian cancer patients is mediated by TNFR2+ Tregs within the tumor microenvironment. Clin. Immunol. 149, 97–110 (2013).
Govindaraj, C. et al. Reducing TNF receptor 2+ regulatory T cells via the combined action of azacitidine and the HDAC inhibitor, panobinostat for clinical benefit in acute myeloid leukemia patients. Clin. Cancer Res. 20, 724–735 (2014).
Yan, F. et al. Expression of TNFR2 by regulatory T cells in peripheral blood is correlated with clinical pathology of lung cancer patients. Cancer Immunol. Immunother. 64, 1475–1485 (2015).
Torrey, H. et al. Targeting TNFR2 with antagonistic antibodies inhibits proliferation of ovarian cancer cells and tumor-associated Tregs. Sci. Signal 10, eaaf8608 (2017).
Saftig, P. & Reiss, K. The “A disintegrin and metalloproteases” ADAM10 and ADAM17: novel drug targets with therapeutic potential? Eur. J. Cell Biol. 90, 527–535 (2011).
Mullooly, M., McGowan, P. M., Crown, J. & Duffy, M. J. The ADAMs family of proteases as targets for the treatment of cancer. Cancer Biol. Ther. 17, 870–880 (2016).
Witters, L. et al. Synergistic inhibition with a dual epidermal growth factor receptor/HER-2/neu tyrosine kinase inhibitor and a disintegrin and metalloprotease inhibitor. Cancer Res. 68, 7083–7089 (2008).
Saha, N., Robev, D., Himanen, J. P. & Nikolov, D. B. ADAM proteases: emerging role and targeting of the non-catalytic domains. Cancer Lett. 467, 50–57 (2019).
Andrews, L. P., Yano, H. & Vignali, D. A. A. Inhibitory receptors and ligands beyond PD-1, PD-L1 and CTLA-4: breakthroughs or backups. Nat. Immunol. 20, 1425–1434 (2019).
Balic, J. J., Garbers, C., Rose-John, S., Yu, L. & Jenkins, B. J. Interleukin-11-driven gastric tumourigenesis is independent of trans-signalling. Cytokine 92, 118–123 (2017).
Berg, A. F. et al. Exclusive inhibition of IL-6 trans-signaling by soluble gp130(FlyR)Fc. Cytokine X 3, 100058 (2021).
Heise, D. et al. Selective inhibition of IL-6 trans-signaling by a miniaturized, optimized chimeric soluble gp130 inhibits T(H)17 cell expansion. Sci. Signal 14, eabc3480 (2021).
Rios-Doria, J. et al. A monoclonal antibody to ADAM17 inhibits tumor growth by inhibiting EGFR and non-EGFR-mediated pathways. Mol. Cancer Ther. 14, 1637–1649 (2015).
Allen-Mersh, T. G., Glover, C., Fordy, C., Henderson, D. C. & Davies, M. Relation between depression and circulating immune products in patients with advanced colorectal cancer. J. R. Soc. Med. 91, 408–413 (1998).
Sakata, H., Murakami, S. & Hirayama, R. Serum soluble interleukin-2 receptor (IL-2R) and immunohistochemical staining of IL-2R/Tac antigen in colorectal cancer. Int J. Clin. Oncol. 7, 312–317 (2002).
Gonda, K. et al. Serum soluble interleukin-2 receptor is increased in malnourished and immunosuppressed patients with gastric and colorectal cancer: possible influence of myeloid-derived suppressor cells. World J. Oncol. 3, 158–164 (2012).
Murakami, S. et al. Serum-soluble interleukin-2 receptor concentrations in patients with gastric cancer. Cancer 74, 2745–2748 (1994).
Murakami, S. et al. Serum soluble interleukin-2 receptor and immunohistochemical staining of IL-2R/Tac antigen in gastric cancer. Oncol. Rep. 3, 69–73 (1996).
Oka, M. et al. Relationship between serum levels of soluble interleukin-2 receptor and various disease parameters in patients with squamous cell carcinoma of the esophagus. Hepatogastro. Enterol. 46, 2254–2259 (1999).
Wang, L. S. et al. Clinical significance of serum soluble interleukin 2 receptor-alpha in esophageal squamous cell carcinoma. Clin. Cancer Res. 6, 1445–1451 (2000).
Gross, M. et al. The diagnostic and prognostic value of sIL-2R as an immune biomarker in head and neck cancers. Anticancer Res. 36, 4347–4352 (2016).
Tartour, E. et al. Soluble interleukin-2 receptor serum level as a predictor of locoregional control and survival for patients with head and neck carcinoma: results of a multivariate prospective study. Cancer 79, 1401–1408 (1997).
Murakami, J. et al. Serum soluble interleukin-2 receptor levels for screening for malignant lymphomas and differential diagnosis from other conditions. Mol. Clin. Oncol. 11, 474–482 (2019).
Stasi, R. et al. The prognostic value of soluble interleukin-6 receptor in patients with multiple myeloma. Cancer 82, 1860–1866 (1998).
Alexandrakis, M. G. et al. Relationship between circulating serum soluble interleukin-6 receptor and the angiogenic cytokines basic fibroblast growth factor and vascular endothelial growth factor in multiple myeloma. Ann. Hematol. 82, 19–23 (2003).
Van Zaanen, H. C. et al. Blocking interleukin-6 activity with chimeric anti-IL6 monoclonal antibodies in multiple myeloma: effects on soluble IL6 receptor and soluble gp130. Leuk. Lymphoma 31, 551–558 (1998).
Jablonska, E. & Pietruska, Z. Changes in soluble IL-6 receptor and IL-6 production by polymorphonuclear cells and whole blood cells of breast cancer patients. Arch. Immunol. Ther. Exp. Warsz. 46, 25–29 (1998).
Jablonska, E. Changes in sIL-6R and sTNF-Rs release by PMNs and the serum levels in breast cancer patients at different stages of treatment. Cytokine 10, 540–543 (1998).
Jablonska, E., Kiluk, M., Markiewicz, W., Piotrowski, L. & Grabowska, Z. Jablonski J. TNF-alpha, IL-6 and their soluble receptor serum levels and secretion by neutrophils in cancer patients. Arch. Immunol. Ther. Exp. Warsz. 49, 63–69 (2001).
Robak, T., Wierzbowska, A., Blasinska-Morawiec, M., Korycka, A. & Blonski, J. Z. Serum levels of IL-6 type cytokines and soluble IL-6 receptors in active B-cell chronic lymphocytic leukemia and in cladribine induced remission. Mediat. Inflamm. 8, 277–286 (1999).
Babic, A. et al. Soluble tumour necrosis factor receptor type II and survival in colorectal cancer. Br. J. Cancer 114, 995–1002 (2016).
Chan A. T., Ogino S., Giovannucci E. L. & Fuchs C. S. Inflammatory markers are associated with risk of colorectal cancer and chemopreventive response to anti-inflammatory drugs. Gastroenterology 140, 799–808 (2011)..
Hamaguchi, T. et al. Exploration of potential prognostic biomarkers in aflibercept plus FOLFIRI in Japanese patients with metastatic colorectal cancer. Cancer Sci. 110, 3565–3572 (2019).
Zeng, F. et al. Inflammatory markers of CRP, IL6, TNFalpha, and soluble TNFR2 and the risk of ovarian cancer: a meta-analysis of prospective studies. Cancer Epidemiol. Biomark. Prev. 25, 1231–1239 (2016).
Nomelini, R. S. et al. TNF-R2 in tumor microenvironment as prognostic factor in epithelial ovarian cancer. Clin. Exp. Med 18, 547–554 (2018).
Dobrzycka, B., Terlikowski, S. J., Kowalczuk, O. & Kinalski, M. Circulating levels of TNF-alpha and its soluble receptors in the plasma of patients with epithelial ovarian cancer. Eur. Cytokine Netw. 20, 131–134 (2009).
Nakamura, N. et al. Serum level of soluble tumor necrosis factor receptor 2 is associated with the outcome of patients with diffuse large B-cell lymphoma treated with the R-CHOP regimen. Eur. J. Haematol. 91, 322–331 (2013).
Goto, N. et al. Serum-soluble tumor necrosis factor receptor 2 (sTNF-R2) level determines clinical outcome in patients with aggressive non-Hodgkin’s lymphoma. Eur. J. Haematol. 77, 217–225 (2006).
Casasnovas, R. O. et al. Plasma cytokine and soluble receptor signature predicts outcome of patients with classical Hodgkin’s lymphoma: a study from the Groupe d’Etude des Lymphomes de l’Adulte. J. Clin. Oncol. 25, 1732–1740 (2007).
Heemann, C. et al. Circulating levels of TNF receptor II are prognostic for patients with peripheral T-cell non-Hodgkin lymphoma. Clin. Cancer Res. 18, 3637–3647 (2012).
Shiels, M. S. et al. Circulating inflammation markers, risk of lung cancer, and utility for risk stratification. J. Natl Cancer Inst. 107, djv199 (2015).
Gregorc, V. et al. Prognostic value of circulating chromogranin A and soluble tumor necrosis factor receptors in advanced nonsmall cell lung cancer. Cancer 110, 845–853 (2007).
Chen, J. et al. Increased serum soluble IL-15Ralpha levels in T-cell large granular lymphocyte leukemia. Blood 119, 137–143 (2012).
Peng, Y. et al. A comprehensive profiling of soluble immune checkpoints from the sera of patients with non-small cell lung cancer. J. Clin. Lab Anal. 36, e24224 (2022).
Zhang, G. et al. Diagnosis value of serum B7-H3 expression in non-small cell lung cancer. Lung Cancer 66, 245–249 (2009).
Mu, C. Y., Qin, P. X., Qu, Q. X., Chen, C. & Huang, J. A. Soluble CD40 in plasma and malignant pleural effusion with non-small cell lung cancer: A potential marker of prognosis. Chronic Dis. Transl. Med. 1, 36–41 (2015).
Zhang, J. et al. Circulating PD-L1 in NSCLC patients and the correlation between the level of PD-L1 expression and the clinical characteristics. Thorac. Cancer 6, 534–538 (2015).
Cheng, H. Y. et al. Circulating programmed death-1 as a marker for sustained high hepatitis B viral load and risk of hepatocellular carcinoma. PLoS One 9, e95870 (2014).
Finkelmeier, F. et al. High levels of the soluble programmed death-ligand (sPD-L1) identify hepatocellular carcinoma patients with a poor prognosis. Eur. J. Cancer 59, 152–159 (2016).
Simone, R. et al. A soluble form of CTLA-4 is present in paediatric patients with acute lymphoblastic leukaemia and correlates with CD1d+ expression. PLoS One 7, e44654 (2012).
Hock, B. D. et al. Identification of a circulating soluble form of CD80: levels in patients with hematological malignancies. Leuk. Lymphoma 45, 2111–2118 (2004).
Rossille, D. et al. High level of soluble programmed cell death ligand 1 in blood impacts overall survival in aggressive diffuse large B-Cell lymphoma: results from a French multicenter clinical trial. Leukemia 28, 2367–2375 (2014).
Guo, X. et al. High serum level of soluble programmed death Ligand 1 is associated with a poor prognosis in hodgkin lymphoma. Transl. Oncol. 11, 779–785 (2018).
Bi, X. W. et al. PD-L1 is upregulated by EBV-driven LMP1 through NF-kappaB pathway and correlates with poor prognosis in natural killer/T-cell lymphoma. J. Hematol. Oncol. 9, 109 (2016).
Acknowledgements
This work was supported by grants (NRF-2022R1A2B5B03001431 and NRF-2022M3A9H1014129) of National Research Foundation (NRF) funded by the Ministry of Education, Science, and Technology (MEST), Republic of Korea.
Author information
Authors and Affiliations
Contributions
E-.J.P. designed and wrote the manuscript. C-.W.L. wrote, supervised, and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Park, EJ., Lee, CW. Soluble receptors in cancer: mechanisms, clinical significance, and therapeutic strategies. Exp Mol Med 56, 100–109 (2024). https://doi.org/10.1038/s12276-023-01150-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s12276-023-01150-6