Abstract
CD8 T cells play crucial roles in immune surveillance and defense against infections and cancer. After encountering antigenic stimulation, naïve CD8 T cells differentiate and acquire effector functions, enabling them to eliminate infected or malignant cells. Traditionally, cytotoxic T cells, characterized by their ability to produce effector cytokines and release cytotoxic granules to directly kill target cells, have been recognized as the constituents of the predominant effector T-cell subset. However, emerging evidence suggests distinct subsets of effector CD8 T cells that each exhibit unique effector functions and therapeutic potential. This review highlights recent advancements in our understanding of CD8 T-cell subsets and the contributions of these cells to various disease pathologies. Understanding the diverse roles and functions of effector CD8 T-cell subsets is crucial to discern the complex dynamics of immune responses in different disease settings. Furthermore, the development of immunotherapeutic approaches that specifically target and regulate the function of distinct CD8 T-cell subsets holds great promise for precision medicine.
Similar content being viewed by others
Introduction
Conventional T cell populations consist of CD4 T cells and CD8 T cells that recognize cognate antigenic peptides presented by MHC class II and I molecules, respectively. Typically, CD4 T cells orchestrate overall immune responses through interactions with professional antigen-presenting cells, whereas CD8 T cells exhibit direct cytotoxicity against target cells. CD8 T cells are derived from lymphoid progenitor cells in the bone marrow and undergo further maturation in the thymus. Thymic mature CD8 T cells, known as naïve CD8 T cells, are poised to be activated after encountering specific antigens. Recent studies have highlighted the phenotypic and functional diversity among naïve CD8 T cells, revealing their heterogeneity1. The cytotoxicity-inducing ability of CD8 T cells was initially discovered in the infectious disease context2,3,4,5,6. After infection, naïve CD8 T cells proliferate and differentiate into effector CD8 T cells, enabling them to efficiently eliminate infected cells and protect the host from severe infection. Following antigen clearance, a fraction of effector CD8 T cells differentiate into memory cells, which can immediately proliferate upon re-exposure to the antigen, ensuring a swift and robust immune response7,8,9,10. However, when CD8 T cells are subjected to persistent antigen stimulation, as seen in chronic viral infections or tumors, they become exhausted, impairing their responses to subsequent antigen stimulation11,12,13.
Extensive research has focused on discerning the cellular biology of CD8 T cells driven by their potent cytotoxicity-inducing capacities, which has significantly advanced the field of tumor immunotherapy. This progress has led to the development of numerous immunotherapeutic strategies that harness the potential of CD8 T cells. The discovery of the cytotoxic function of CD8 T cells has paved the way for innovative therapeutic approaches. One prominent approach is adoptive cell therapy, which involves the isolation of CD8 T cells from patients and their subsequent ex vivo expansion and activation before being reintroduced back into the patient14,15. Specifically, significant strides have been made in the field of chimeric antigen receptor (CAR)-T-cell therapy, which involves genetically engineered patient CD8 T cells that effectively recognize cancer antigens. To date, the FDA has approved and clinically utilized more than six types of CAR-T-cell therapies for the treatment of certain types of hematologic malignancies16,17. Additionally, immune checkpoint inhibitors have emerged as a groundbreaking immunotherapeutic strategy that unleashes the full potential of CD8 T cells in response to tumors. By blocking inhibitory receptors, such as programmed cell death protein-1 (PD-1) or cytotoxic T-lymphocyte-associated protein-4 (CTLA-4), inhibitors restore the activity of CD8 T cells and promote their antitumor responses. This approach has shown remarkable success in certain types of cancer and has revolutionized the field of cancer immunotherapy.
Although many studies have traditionally been focused on cytotoxic CD8 T cells, also known as Tc1 cells, which are characterized by their production of cytolytic cytokines such as IFN-γ, granzyme B, and TNF-α, recent investigations have revealed additional subsets of effector CD8 T cells, each with distinct characteristics that differ from those of Tc1 cells. The cells in these subsets demonstrate reduced cytotoxicity but produce a diverse array of cytokines and express transcription factors that are similar to those expressed in CD4 T-cell subsets, suggesting that their effects extend beyond directly induced cytotoxicity. These cells play a pivotal role in triggering cytokine-mediated effector functions and actively participate in immune regulation and tissue homeostasis maintenance. Furthermore, they may contribute to the modulation of immune responses, the orchestration of inflammatory processes, and the coordination of immune cell interactions.
Cytotoxic CD8+ T cells (Tc1 cells)
As typical cytotoxic CD8+ T cells, Tc1 cells produce perforin, granzyme B, IFN-γ, and TNF-α, which enable them to eliminate tumor and infected cells. The activation of Tc1 cells is promoted by IL-12, which is produced by antigen-presenting cells exposed to pathogen-derived maturation-promoting stimuli. Several key transcription factors, such as STAT4, T-bet, and EOMES, contribute to the polarization of Tc1 cells14,18. Activated Tc1 cells have traditionally been thought to kill tumor or infected cells through mechanisms involving perforin-granzyme and Fas-FasL signaling. However, recent studies suggest that Tc1 cells kill target cells through additional cytotoxic pathways, including ferroptosis and pyroptosis19.
Tc1 cells constitute the most prevalent subset of tumor-infiltrating lymphocytes in multiple types of cancers, including lung cancers20, breast cancers21, and chronic lymphocytic leukemia22, and are associated with favorable prognoses23,24,25,26,27. Tumors exhibiting a high degree of infiltration by Tc1 cells that subsequently produced elevated levels of IFN-γ are referred to as “hot” tumors. These “hot” tumors exhibit a more favorable response to immunotherapies than “cold” tumors, which lack Tc1 cell infiltration28,29. The expression of CD29 is associated with the increased cytotoxic potential of Tc1 cells in melanoma patients, suggesting that CD29 is a novel marker for the cytotoxic potency of Tc1 cells30.
The significance of Tc1 cells has also been established in the viral disease contexts, including patients infected with measles virus, cytomegalovirus, hepatitis C virus, and human immunodeficiency virus (HIV)31,32,33,34. Following the clearance of infected cells, effector Tc1 cells can differentiate into memory cells7. Memory Tc1 cells retain the cytotoxic phenotype of effector Tc1 cells35 and readily produce IFN-γ upon reactivation36. Even in antigen-naive mice, a population of memory-like Tc1 cells, namely, CD44hiCD122hiCD8+ T cells, which are different from naïve T cells, can rapidly produce IFN-γ in response to TCR stimulation37,38,39. These cells exhibit a distinct epigenetic pattern on the Ifng promoter, suggesting that cytokine production may be regulated at the transcriptional level independent of previous antigen exposure-induced cell priming. Memory Tc1 cells are crucial for protection against cancer and infection40,41. In particular, stem cell-like memory T cells show superior antitumor immunity compared to effector T cells40. Moreover, memory Tc1 cells are relatively unaffected by the inhibitory effects of regulatory T cells (Tregs) in the tumor microenvironment42. Thus, the frequency of memory Tc1 cells has been proposed to be a potential predictive marker for responsiveness to ICI treatment43,44,45.
Persistent antigen stimulation induces a state of exhaustion in effector Tc1 cells, leading to impaired cytolytic molecule production11. A single-cell transcriptome analysis revealed TOX as a key exhaustion-promoting transcription factor in human cancer46. Exhausted Tc1 cells display upregulated protein expression of TOX, which upregulates the protein expression of inhibitory receptors such as PD-1, LAG3, 2B4, and CD3947,48. The degree of Tc1 cell exhaustion is associated with poor outcomes for cancer patients49. Genetic engineering approaches have been used to demonstrate that the overexpression of BATF and IRF4 negatively regulated the expression of TOX in CD8 T cells, resulting in the generation of potent antitumorigenic CD8 T cells within the tumor microenvironment50. Compared to terminally exhausted PD-1hiTim3+TOX+ Tc1 cells, progenitor exhausted PD-1intCXCR5+TCF-1+ Tc1 cells exhibited better control of tumor growth12,51,52. In contrast to terminally exhausted Tc1 cells, progenitor exhausted Tc1 cells are responsive to ICI immunotherapy12,51. Therefore, study into ways to reinvigorate exhausted Tc1 cells is an important research direction, as these cells hold great promise for developing novel immunotherapeutic approaches to chronic infections, cancers, and other diseases associated with Tc1 cell dysfunction.
Non-Tc1 cell CD8 T-cell subsets
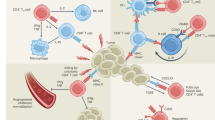
Although Tc1 cells represent the major population of CD8 T cells, alternative CD8 T-cell subsets have been identified in various diseases in both animal and human models (Fig. 1). CD8 T-cell subsets closely resemble their CD4 T-cell subset counterparts, as both CD4 T cells and CD8 T cells share similar signal 3 cytokine requirements, lineage-determining transcription factors, and effector cytokine profiles (Fig. 2, Table 1).
Tc subsets play diverse roles in different diseases. Tc1 cells are known for their cytotoxic activity against tumors and infected cells, while Tc2 cells have been implicated in the pathogenesis of allergic diseases and cancer. Tc17 cells have been studied in the context of skin inflammation and the tumor microenvironment, while Tc9 cells have been associated with certain autoimmune disorders and antitumor effects. The role of Tc22 cells has been reported in psoriatic skin and tumor tissue. Tfcs support humoral responses in autoimmunity and are also found in the tumor microenvironment. Additionally, regulatory cell subsets such as CD8+ Foxp3+ Tregs, Qa1-restricted CD8+ Tregs and human KIR+ CD8+ Tregs have been identified in graft-versus-host disease and autoimmune diseases, in which they presumably contribute to immune regulation.
Th cell subsets and Tc subsets share signal 3 cytokines, lineage-determining transcription factors, and effector cytokine profiles. Tc1 cells can be differentiated under IL-12 conditions and produce IFNγ through activation of the transcription factors T-bet, Eomes and STAT4. Tc2 cells can be differentiated under IL-4 conditions and produce IL-4, IL-5 and IL-13 through GATA-3 and STAT6. Tc9 cells can be differentiated under IL-4 plus TGF-β conditions and produce IL-9 through IRF-4 and STAT6. Tc17 cells can be differentiated under IL-6 plus TGF-β conditions through RORγt and STAT3. Tc22 cells can be differentiated under IL-6 plus TNF-ɑ conditions and produce IL-22 and TNF-ɑ through AhR, STAT1, STAT3 and STAT5. Tfcs can be differentiated under IL-6, IL-21, and IL-23 plus TGF-β conditions through BCL-6, TCF-1, E2A and Runx3. Foxp3+ CD8+ Tregs can be differentiated under TGF-β conditions and produce IL-10 and TGF-β through Foxp3. Qa-1-restricted CD8+ Tregs recognize the Qa-1 peptide on MHC class I molecules and suppress Qa-1-expressing T cells by secreting perforin meditated through the Eomes signaling pathway.
Tc2 cells
A subset of CD8 T cells producing Th2 cytokines, termed Tc2 cells, has been found in airway and intraepithelial tissues53,54. In vitro stimulation of naïve CD8 T cells with IL-4 induced IL-4- and IL-5-producing CD8 T cells55,56. Th2 cell cytokine production by Tc2 cells was promoted by the transcription factors STAT6 and GATA3, similar to effect of these transcription factors in Th2 cells14. Similar to Th2 cells, Tc2 cells stimulate B-cell IgE production, recruit eosinophils, and contribute to allergic responses57,58.
In the context of allergic asthma, the number of Tc2 cells is increased in severe eosinophilic asthma in humans58. Interestingly, Tc2 cells produce type 2 cytokines in response to stimulation by PGD2 and LTE4, which are major lipid mediators released by mast cells during an allergic response58,59. Compared to Th2 cells, Tc2 cells are less responsive to corticosteroid treatment, highlighting the potential of Tc2 cells as a therapeutic target for steroid-resistant asthma57,60. In steroid-resistant asthma, Cyp11a1, a mitochondrial enzyme that cleaves side chains, has been identified as a key regulator in the differentiation of Tc2 cells61. Moreover, the pathogenicity of Tc2 cells appears to be heightened in a hypoxic environment, as evidenced by the increased production of IL-1362. Similarly, in allergic rhinitis, CD8 T cells release IL-4 and contribute to the pathogenesis of disease63. After allergen-induced immunotherapy, the percentage of IL-4-producing CD8 T cells tends to be significantly reduced in patients with intermittent allergic rhinitis64,65.
Individuals with allergic dermatitis (AD), similar to those with asthma, present with a higher frequency of Tc2 cells66,67. In healthy individuals, Tc2 cells make up ~1% of CD8 T cells, but this proportion is increased to ~4% in AD patients68. Histamine, a potent inflammatory mediator, promotes cross-presentation of antigens by dendritic cells and thus induces the accumulation of Tc2 cells69,70,71. Single-cell RNA sequencing and proteomic analysis of AD patients who had been treated with the IL-4Ra-blocking antibody dupilumab revealed the presence of Tc2 cells in the skin that were not detected in healthy controls, indicating the persistence of tissue-resident memory Tc2 cells72.
Although effector memory Tc1 cells are capable of producing high levels of IFN-γ and cytotoxic granules, effector memory Tc2 cells lack this ability and are ineffective in killing target cells73, suggesting that skewing Tc1 cell transition into Tc2 cells can compromise the antitumor function of CD8 T cells. In cervical cancer patients, tumor cells promote the acquisition of a Tc2 cell phenotype by tumor-infiltrating CD8 T cells, which leads to increased production of IL-4 and decreased production of IFN-γ, facilitating immune escape of tumor cells74. Similarly, in urothelial bladder cancer, the exhaustion and reduced cytotoxicity of CD8 T cells in sentinel nodes are attributed to a decline in perforin expression caused by the Tc2 cell-polarized tumor microenvironment, which leads to an exhausted effector memory phenotype75.
Tc9 cells
IL-9-producing CD8 T (Tc9) cells are transcriptionally regulated by STAT6 and IRF4, which are transcription factors of Tc2 and Th9 cells, respectively14. Stimulation of naïve CD8 T cells in the presence of IL-4 and TGF-β induces the differentiation of Tc9 cells in vitro76. Functionally, both Tc2 and Tc9 cells are implicated as pathogenic drivers of allergic conditions such as allergic asthma and atopic dermatitis57,76. Although not as extensively investigated as Tc2 cells, Tc9 cells are also linked to eosinophilia and elevated levels of FeNO (fractioned exhaled nitric oxide), a noninvasive marker of inflammation in asthma patients77. Although the transfer of Tc9 cells alone is insufficient to induce symptoms of asthma, their co-transfer with Th2 cells results in severe airway inflammation is characterized by an increased number of eosinophils in bronchoalveolar lavage (BAL) and an elevated lung inflammatory score76. In addition, the number of Tc9 cells is also increased in atopic dermatitis in both mice and humans76.
Importantly, Tc9 cells have been identified within the tumor tissue of breast cancer patients, and there is a positive correlation between the transcription levels of IL9 and IL9R78. In contrast to that of Tc2 cells, the adoptive transfer of Tc9 cells led to potent antitumor effects in animal models79. A transcriptome analysis suggested that Tc9 cells in the tumor undergo transcriptional modifications related to cholesterol synthesis and efflux. Mechanistically, the activation of liver X receptor by oxidized cholesterol negatively regulated the differentiation and antitumor activity of Tc9 cells80. A recent study demonstrated that lipid peroxidation plays a crucial role in regulating the stability of Tc9 cells and their antitumor activity by regulating the IL-9-STAT3-fatty acid oxidation axis81. The emerging understanding of Tc9 cells as key players in allergic conditions, asthma pathogenesis and cancer immunity may open new avenues to therapeutic interventions.
Tc17 cells
Tc17 cells are defined as CD8 T cells that produce IL-17 and express the transcription factors STAT3 and RORγt82. In different research contexts, there is ongoing debate among researchers regarding the characteristics of Tc17 cells. Some argue that Tc17 cells express T-bet, which is the master regulator in Th1 and Tc1 cells83. However, opposing arguments have suggested that Tc17 cells exhibit limited cytolytic activity and express minimal levels of granzyme B and perforin, thereby distinguishing them from Tc1 effector cells84. Depending on the disease status and bodily location, Tc17 cells can secrete other cytokines, such as IL-22, GM-CSF, IL-5, and IL-1385,86.
The heterogeneity in cytokine profiles and transcription factors may be partly due to the high plasticity of Tc17 cells (Fig. 3). Multiple studies have confirmed the minimal cytolytic activity of Tc17 cells generated in vitro87,88,89. Interestingly, when in vitro-generated Tc17 cells were adoptively transferred in vivo, they lost IL-17 expression and acquired a Tc1-like phenotype and function; therefore, they secreted IFN-γ and granzyme B89. The activation of the PI3K/AKT pathway has been suggested to play a crucial role in the transdifferentiation of Tc17 cells into Tc1 cells, with Eomes upregulation being an essential component of this reprogramming process90. Furthermore, the commensal bacteria-specific response of Tc17 cells has been shown to drive these cells to acquire a Type 2 transcriptome that can be awakened after tissue injury and subsequent production of alarmins, such as IL-1, IL-18, IL-25, and IL-3386. On the other hand, the plasticity of Tc17 cells was negatively regulated by immune checkpoints, specifically PD-1 and CTLA-491,92. The cross linking of CTLA-4 on Tc17 cells enhances their ability to maintain a Tc17 cell profile and resist transitioning into Tc1 cells, even under Tc1-transdifferentiation-skewing conditions91. Similarly, PD-1 signaling suppressed not only the differentiation of Tc17 cells but also the conversion of Tc17 cells into Tc1 cells, thereby reducing the cytotoxic potential of the overall Tc1 cell population92. The various signaling pathways involved in Tc17 polarization explain the heterogeneity and plasticity of Tc17 cells.
Tc17 cells, akin to Th17 cells, display high plasticity in terms of cytokine production under specific conditions, and conversely, other Tc subsets can transform into Tc17 cells within certain contexts. Adoptive transfer of in vitro differentiated Tc17 cells led to their production of IFN-γ and granzyme B, similar to Tc1 cells. CTLA-4 plays a role in shifting the programming of Tc1 cells toward Tc17 cell differentiation. In the skin, alarmins such as IL-1, IL-18, and IL-33 stimulate commensal bacteria-specific Tc17 cells to produce Tc2 cytokines. Tc17 cells and Tc22 cells exhibit shared features, including similar differentiation-inducing cytokines, signaling transcription factors and cytokine production profiles. In a GvHD model, some adoptively transferred Foxp3+CD8+ Tregs were transformed into Tc17 and Tc1 cells. In addition to Tc17 cells, constituting another Tc subset also exhibit plasticity. Tc1 cells can produce IL-10 and exhibit suppressive functions in the context of viral infection. Tc2 and Tc9 cells share common features, including differentiation-inducing cytokines, signaling transcription factors and cytokine production profiles. In the tumor microenvironment, Tc9 cells undergo conversion to Tc1 cells, leading to potent antitumor immune responses, whereas Tc2 cells do not significantly contribute to attenuated tumor development.
Tc17 cells induce the production of antimicrobial peptides and chemokines, which attract neutrophils to sites of infection or inflammation, thereby contributing to immune defense responses against pathogens such as bacteria and fungi93,94. However, an imbalance or overabundance of Tc17 cells can lead to the pathogenesis of autoimmune inflammatory diseases, including but not limited to Crohn’s disease and psoriasis. In Crohn’s disease, a chronic inflammatory bowel disease that primarily impacts the gastrointestinal tract, Tc17 cells, which normally contribute to gut health by aiding in repair and barrier function, demonstrate altered gene expression patterns associated with cytotoxicity and heightened production of the inflammatory cytokine TNF-α. As expected, patients with Crohn’s disease exhibit a higher frequency of Tc17 cells than healthy individuals95. Moreover, high-dimensional profiling studies identified enrichment of Tc17 cells in active Crohn’s disease samples, specifically highlighting a subset of cells expressing CD6high, CD39, CD69, PD-1, and CD27low, which may serve as targets for therapeutic interventions96.
In psoriasis, Tc17 cells are key contributors to disease onset, arguably playing a more prominent role than Th17 cells85,97,98,99. A markedly increased number of Tc17 cells are recruited to psoriatic lesions compared to the number in normal skin, whereas no significant difference has been found in Th17 cell levels. Moreover, the frequency of Tc17 cells in the peripheral blood of psoriasis patients correlated with disease severity99. A single-cell transcriptomic analysis identified distinct inflammatory Tc17 cell subsets enriched in psoriatic lesions that commonly express CXCL13, a biomarker of psoriasis severity98.
Memory Tc17 cells possess both tissue-resident and effector memory properties. Despite their high plasticity as effector cells, as antifungal vaccine-induced memory cells, Tc17 cells persisted in maintaining a stable type 17 phenotype and were not converted into Tc1 cells100. Most memory Tc17 cell with activation induced by an antifungal vaccination expressed CD103, an integrin and marker of tissue-resident memory cells, and high levels of CD127 (IL-7Ra); thus, sharing similar phenotypes with tumor-infiltrating Tc17 cells while also expressing phenotypic markers, namely, CD62Llo and Ly6Clo, that are consistent with effector memory cells87.
The roles of Tc17 cells in the tumor microenvironment is still debated (Fig. 4). Memory Tc17 cells, with enhanced self-renewal abilities and potential for long-lasting memory, are considered promising candidates for cancer immunotherapy89. In contrast to the stable Tc17 cells observed after fungal vaccination, in vitro-generated Tc17 cells were easily converted into Tc1 cells in tumor environments; therefore, they served as a reservoir of Tc1 cells in vivo89. On the other hand, in vivo-generated Tc17 cells exhibited a stable phenotype and have been shown to promote the exhaustion of Tc1 cells, presumably by recruiting CD11b+Gr-1+ MDSCs in animal models87. Additionally, a specific subset of Tc17 cells, characterized by low PD-1 expression and high OX40 expression, has been associated with reduced patient survival rates83. In hepatitis B virus infection, IL-17 attracted CD11b+Gr-1+ MDSCs to trigger CD8 T-cell exhaustion101, whereas in myelodysplastic syndromes, CD11b+Gr-1+ MDSCs have been proposed to induce CD8 T-cell exhaustion via the Tim-3/Galectin-9 pathway102.
Tc17 cells in the tumor microenvironment play distinct roles in tumor growth depending on their origin. ① In vitro-generated Tc17 cells are converted to effector Tc1 cells and repress tumor cell growth. On the other hand, Tc17 cells generated in vivo have been shown to exhibit pro-tumorigenic effects. ② Tc17 cells induced in the tumor microenvironment followed by CD4 depletion to recruit infiltrating myeloid cells and accelerate the exhaustion of effector Tc1 cells. ③ Tc17 cells from cancer patients presented with a terminally exhausted phenotype, which resulted in impaired antitumor immune responses.
Tc22 cells
Tc22 cells, constituting a less-extensively investigated subgroup of CD8 T cells, are known for their production of IL-22. IL-22 belongs to the IL-10 family of cytokines and primarily targets epithelial cells, keratinocytes, hepatocytes, and pancreatic β cells103. Similar to IL-17, IL-22 maintains the epithelial barrier by promoting tissue repair and wound healing104. Tc22 cells share similarities with Tc17 cells, as they produce a small amount of the IL-17 cytokine, and Tc17 cells produce IL-2214. Moreover, the cytokine requirements for the in vitro differentiation of Tc22 cells resemble those of Tc17 cells; that is, they require the IL-6 and IL-21 cytokines103,105. In line with this, there have been reports of IL-17 and IL-22 being overrepresented in coproducing CD8 T cells in psoriatic lesions85, raising questions about whether Tc22 is truly a distinct T-cell line. However, further inspection of dermal samples revealed an increase in IL-22-producing CD8 T cells that lacked IL-17 expression in psoriatic lesions, reinforcing the idea that Tc22 cells constitute a separate T-cell subset85.
A recent investigation has shed light on the role of Tc22 cells in the context of tumors. In vitro-generated Tc22 cells exhibited high cytolytic activity and effective control of tumor growth when transferred into tumor-bearing hosts, and their effects were comparable to or even more pronounced than those of Tc1 cells103. Intriguingly, Tc22 cells have been detected in populations of tumor-infiltrating lymphocytes (TILs) from ovarian cancer tissue that had been expanded, thereby comprising up to ~35% of the CD8 T cells in some patients, and the production of IL-22 by these CD8+ TILs correlated with increased recurrence-free survival. This finding suggested that directing T cells toward differentiation into a Tc22 cell lineage may hold promise for advancing cell therapies such as CAR-T-cell or TCR-based immunotherapy103. In transplant-associated squamous cell carcinoma (TSCC), however, an increase in the number of Tc22 cells was associated with a decrease in the number of Th1 cells and an increase in tumor growth, suggesting that Tc22 cells may contribute to the progression of TSCC106.
In the context of viral infection, individuals exposed to HIV but not infected with the virus exhibited a higher frequency of Tc22 cells compared to their HIV-infected partners, who tended to produce a relatively higher proportion of Tc17 cells, suggesting the protective role of Tc22 cells against viral infections such as HIV107. In the acute phase of SARS-CoV-2 infection, which causes COVID-19, an observed increase in the frequency of Tc22 cells compared to that in healthy control groups has been observed. Tc22 cells have been associated with milder symptoms or even asymptomatic cases, suggesting a potential protective effect against SARS-CoV-2 infection108.
Follicular cytotoxic T cells (Tfcs)
Follicular cytotoxic T cells (Tfcs) are a recently explored subset of CD8 T cells. Similar to follicular helper T cells (Tfh cells), Tfc cells express the chemokine receptor CXCR5, which facilitates their migration to the germinal center. The differentiation of Tfc cells is induced by the cytokines IL-6, IL-21, IL-23, and TGF-β, which activate multiple transcription factors, including TCF-1, BCL-6, E2a, and Runx3, playing important roles in the differentiation of Tfc cells109,110. Extensive research has been conducted to investigate the role of Tfc cells in both the humoral response and the antitumor response.
Tfc cells were initially discovered in the B-cell follicle of human tonsils111. When cocultured with B cells, Tfc cells enhance both the survival and antibody production of B cells111. IL-2 knockout mice, which lack regulatory T cells and are susceptible to autoimmune diseases, exhibited a notable increase in the population of Tfc cells. Notably, depletion of CD8 T cells led to a decrease in B-cell frequency and autoantibody production and an increase in survival rate. These findings indicate that Tfc cells play a crucial role in enhancing B-cell responses in vivo112. Tfc cells not only function as B-cell helpers themselves but also synergistically cooperate with Tfh cells to facilitate B-cell maturation and antibody class switching112,113. Mechanistically, Tfc cells support B-cell function through the secretion of IL-21 and CD40L113. The increased antibody production by Tfc cells in human and mouse models has prompted clinical investigations into the correlation between Tfc cell frequency and the severity of viral infections. In HIV infection, it has been reported that the quantity of Tfc cells increases in HIV-infected patients compared to healthy controls, and their frequency is inversely correlated with the viral load of HIV114,115,116. In contrast to the B-cell helper function of Tfc cells, recent studies have revealed a distinct population of IFN-ɣ+CXCR5+ Tfc cells that exert suppressive effects on antibody production in both mouse and human models117,118. In the transplantation model, the presence of IFN-ɣ+CXCR5+ Tfc cells inhibits alloantibody production117. The suppressive function of these cells is dependent on IFN-ɣ, as demonstrated by the inability of IFN-ɣ-deficient CD8 T cells to suppress antibody production119.
In addition to their role in regulating antibody responses, the role of Tfc cells has been reported in several cancer studies. Tfc cells are found in a wide range of tumors, including both solid tumors, such as non-small cell lung cancer, and liquid tumors, such as follicular B-cell non-Hodgkin’s lymphoma120,121,122. Tfc cells in the tumor microenvironment are characterized by their expression of TCF1 and PD-1 and low levels of TIM3. These cells exhibit a less cytotoxic phenotype than effector cells and share similarities with progenitor exhausted Tc1 cells, displaying limited proliferation and cytokine production123,124. Additionally, Tfc cells possess self-renewal capacity and responsiveness to immune checkpoint blockade, which are characteristics of progenitor exhausted Tc1 cells125,126. These progenitor exhausted phenotypes of Tfc cells suggest their potential as promising therapeutic targets in cancer treatment. Clinical studies have revealed a correlation between the frequency of Tfc cells and disease-free or overall survival in various cancers, including pancreatic, colon, follicular lymphoma, gastric, high-grade serous ovarian, hepatocellular, and bladder cancers127,128,129,130,131,132. Collectively, Tfc cells are emerging as a potential biomarker for predicting prognosis in both viral infections and cancer.
CD8+ Tregs
Regulatory T cells are crucial for maintaining immune homeostasis and preventing autoimmune disorders. Although CD4+ Foxp3+ cells are recognized as predominant regulatory T cells, multiple studies have demonstrated that CD8+ Tregs play immune-suppressing roles in diverse human and murine systems133,134,135.
The suppressive functions of CD8 T cells, restricted to the nonclassical MHC class I molecule Qa-1, in humoral responses was discovered in β2m-knockout mice134,136. These suppressive CD8+ Tregs recognize the Qa-1 peptide and are characterized by their expression of CD122 and Ly49 molecules. The suppressive activity of Qa-1-restricted CD8+ Tregs depends on the IL-15 signaling pathway137,138. Qa-1-restricted CD8+ Tregs are essential for self-tolerance mediated through the inhibition of Qa1+ follicular helper T cells, and Helios is required for the terminal differentiation of these cells138,139,140. Recent studies have provided evidence highlighting the essential roles of TGF-β and Eomes in maintaining the homeostasis of Qa-1-restricted CD8+ Tregs, each with distinct contributions. TGF-β signaling maintains the stability of Qa-1-restricted CD8+ Tregs by upregulating Helios expression, and the genetic program governed by Eomes directs the localization of these cells to the germinal center141. Along with lupus-like autoimmune disease, Qa-1-restricted CD8 Tregs directly regulated autoreactive CD4 T cells in an experimental autoimmune encephalomyelitis (EAE) model142,143,144,145,146. Specifically, Qa-1-deficient CD4 T cells escaped the suppressive effects of Qa-1-restricted CD8 Tregs and developed EAE142.
Suppressive Qa-1-restricted CD8+ Tregs have also been identified in humans, and their frequency is highly increased in patients with autoimmune diseases as well as those infected with SARS-CoV-2virus147,148,149. Suppressive human CD8+ Tregs express killer immunoglobulin-like receptors (KIRs), which are the evolutionary equivalent to Ly49 receptors in mice. Patients with autoimmune diseases present with increased KIR expression in T cells. KIR+ CD8 T cells efficiently eliminate gliadin-specific CD4 T cells from celiac disease patients. The frequency of KIR+ CD8 T cells is increased in virus-infected patients, including those affected by SARS-CoV-2 and influenza, and this increase in virions is associated with the severity of the disease. Notably, TCR sequencing analysis revealed that KIR+ CD8 T cells share a common TCR repertoire and antigen specificity that is independent of disease type148.
The other subset of regulatory CD8 T cells, known as Foxp3+CD8+ Tregs, can be induced during early graft-versus-host disease (GVHD). Through transcriptional profiling, it has been discovered that Foxp3+CD8+ Tregs exhibit a transcriptional signature consistent with canonical Foxp3+CD4+ Tregs and actively contribute to the suppression of GVHD by supporting Foxp3+CD4+ Treg function. Notably, in the absence of Foxp3+CD4+ Tregs, Foxp3+CD8+ Tregs efficiently prevent GVHD-induced severe inflammation, demonstrating their potent regulatory capabilities150,151,152. Surprisingly, 60% of adoptively transferred Foxp3+CD8+ Tregs produced IFN-γ, while 20% of these cells produced IL-17 in a GVHD model152. In vitro-differentiated CD103+Foxp3+CD8+ Tregs suppressed CD4 T-cell responses and ameliorated CD4 T-cell-mediated lupus nephritis by directly suppressing B-cell responses153,154. These findings collectively underscore the essential role of CD8+ Tregs in self-tolerance and highlight their potential significance in autoimmune disorders and viral infections such as SARS-CoV-2 (Fig. 5).
Suppressive CD8+ Tregs are distinguished by the expression of Foxp3. Qa-1-restricted CD8+ T cells and KIR+ human CD8+ T cells do not express Foxp3. Qa-1-restricted CD8+ T cells recognize the Qa-1 peptide on MHC class I molecules of autoreactive Tfh cells and suppress autoimmune diseases. Suppressive human CD8 T cells express KIR, which is the genetic counterpart of the Ly49 molecule in mice, and suppress autoreactive CD4 T cells that can cause tissue damage in autoimmune disorders. Foxp3-expressing CD8 T cells are activated in an early GVHD model in vivo and can be converted into IFN-γ and IL-17A-producing CD8 T cells.
Therapeutic potential of Tc subsets
Tc1 cells form the backbone of the cancer immunotherapies that have been applied successfully to date. More than seven ICI therapies155,156,157,158,159,160,161 and six CAR-T-cell therapies162,163,164,165,166,167 have been approved by the FDA for several types of malignancies. To improve the therapeutic outcome of cancer immunotherapy, combinations consisting of ICIs with other immuno-oncology agents have been evaluated168. Specifically, combining ICIs with an adenovirus-based tumor antigen vaccine, an IL-15 superagonist (N-803), an anti-OX40/4-1BB, and docetaxel has demonstrated therapeutic benefits in both hot and cold tumor models, synergistically triggering an immune response169. Furthermore, the success of CAR-T-cell therapy has encouraged researchers to develop similar treatments for diseases in addition to cancer17. Second-generation CARs targeting HBV-surface proteins S and L (HBV infection), fibroblast activation protein (fibrotic disease), CD19 or autoantigens (autoimmune diseases), or uPAR (senescence) have been invented, and clinical trials for these CAR-T-cell therapies are ongoing16,17.
Targeting Tc2 cells is a novel and appealing strategy for treating asthma patients. Specifically, a human sample study showed that TM30089 and montelukast, which block PGD2 receptors and leukotriene receptors of Tc2 cells, respectively, reduced IL-5/IL-13 production and attenuated the migration of Tc2 cells that had been induced by PGD2 and LTE458. Clinically, fevipiprant, a PGD2 receptor 2 antagonist, demonstrated therapeutic advantages in specific subsets of asthma patients in Phase 2 trials170,171. Although Phase 3 studies failed to yield a statistically significant result, a subtle but consistent reduction in the rates of exacerbation was observed with increasing dosages of fevipiprant172. In addition, several studies showed that HIF-1α inhibitors reduced the hypoxia-enhanced differentiation of Tc2 cells and allergic responses in lungs62,173. Moreover, aminoglutethimide and vitamin D3 downregulated the enzymatic activity of CYP11A1, leading to reduced IL-4-mediated conversion of CD8 T cells to IL-13-secreting pathogenic CD8 T cells61,174. Adding vitamin D to a treatment regimen led to a statistically significant reduction in asthma exacerbation rates in adult asthma patients with low levels of vitamin D, although there has been no consistent evidence of this effect in the pediatric population175. Omalizumab, a recombinant anti-IgE monoclonal antibody that has demonstrated efficacy in asthma treatments176, has been found to reduce not only the IgE concentration but also significantly reduced the frequency of IL-13-secreting CD8 T cells in patients with allergic asthma177. In cases of dermatitis, blockade of histamine receptors using thioperamide or JNJ7777120 decreased hapten-induced local inflammation and IL-13 secretion by CD8 T cells, with a more pronounced inhibitory effect than that of CD4 T cells69. Oclacitinib, a Janus kinase inhibitor indicated for pruritus or atopic dermatitis in dogs178, has shown a significant reduction in Tc2 and Th2 cells, and this treatment effect was more pronounced for Tc2 cells than for Th2 cells179. Allergen immunotherapy, another potential strategy, has been shown to significantly reduce the intracellular expression of IL-4 by Tc2 cells in the context of intermittent allergic rhinitis64. A tendency toward reducing the ratio of Tc2 cells/Tc1 cells was observed, while no significant changes in the ratio of Th2 cells/Th1 cells was observed.
Tc9 cells show superior effector function for in the context of adoptive cancer immunotherapy. Compared to Tc1 cells, Tc9 cells showed less cytolytic activity in vitro but surprisingly elicited greater in vivo antitumor responses against advanced tumors in model mice79. In fact, TNF-α-induced tumor-specific Tc9 cells displayed enhanced antitumor capabilities compared to the those of control Tc9 cells. These enhanced abilities involved increased cell survival and proliferation mediated by STAT5 or nuclear factor-κB signaling180. Additionally, adoptive transfer of Tc9 and Th9 cells has shown led enhanced antileukemic activity compared with that of Tc1 cells while attenuating the severity of GVHD, thereby reinforcing the suggestion that Tc9 cells are attractive cancer immunotherapy targets181.
As Tc17 cells play crucial roles in the pathogenesis of autoimmune diseases and cancer, targeting Tc17 cells may be a promising therapeutic strategy. Ustekinumab, an IL-12/IL-23 inhibitor, led to a substantial and persistent reduction in peripheral blood Tc17 and Th17 cells, and this effect was accompanied by clinical improvement of cutaneous and mucosal lesions in Lichen Planus patients182. Furthermore, treatment with ursolic acid (UA), a small-molecule inhibitor of RORγt, has been shown to efficiently repress the exhaustion of CD8+ TILs and reduce the tumor burden in tumor-bearing mice87. In human metastatic melanoma cancer cells, UA exhibited potent antitumor effects183. A Phase I trial was conducted to assess the safety of multiple doses and evaluate the antitumor effect of UA184. Dimethyl fumarate (DMF), indicated for multiple sclerosis and psoriasis, has been observed to inhibit the production of IL-17 by Tc17 cells and thus alter the transcriptional profile, including increased IFN-γ secretion, to be similar to that of “CTL-like” cells185.
Tc22 cells play a role in eliminating cancer cells. Specifically, the administration of pantothenate, a CoA precursor known to enhance IL-22 production by Tc22 cells, in combination with immune checkpoint inhibitor (ICI) therapy has demonstrated promising outcomes by eliminating tumors in mice186,187. Although the adoptive transfer of both Tc1 and Tc22 cells exerts antitumor effects, on average, mice receiving Tc22 cells showed prolonged survival compared to that of mice that received Tc1 cells103. Thus, it might be prudent to polarize T cells into the Tc22 lineage when utilizing CAR- or TCR transduction-based T-cell immunotherapies.
Although the utilization of CD8+ Tregs in the clinic has not been realized thus far, mainly due to the challenges in their identification and characterization, there is growing interest in exploring various agents that can modulate CD8+ Tregs in the context of relevant diseases188. Blocking CD40/CD40L and ICOS/B7h led to potent induction of CD8+CD45RClow/− Tregs and CD8+PD1+ Tregs, respectively, inducing tolerance in vivo in mice with GVHD189,190. Another study showed that anti‐CD45RC mAbs depleted CD45RChi naïve and effector memory T cells re-expressing CD45RA (TEMRA) but preserved the abundance CD45RClo Tregs, thereby inducing transplant tolerance efficiently in rats and humanized immune mice191. Interestingly, anti-CD3 mAbs have exhibited efficacy in promoting Foxp3+ CD8+ Treg activity and sustained amelioration of RA in mice and Type 1 diabetes mellitus in humans192,193. Additionally, IL-2 in combination with TGFβ as well as IL-34 alone have demonstrated the ability to expand both CD4+ Tregs and CD8+ Tregs, which may be leveraged to mitigate diseases such as lupus and GVHD194,195. Moreover, adoptive cell transfer of human CD8+ Tregs and their activation induced by TGF-β1 and rapamycin in mice with collagen-induced arthritis significantly reduced the levels of anti-collagen IgG antibody, clinical scores, and degree of cartilage destruction153. Additionally, human CD8+ CAR-Tregs have been shown to enhance the suppression of human skin rejection and GVHD in NSG mice196, suggesting a potential benefit of CD8+ CAR-Tregs in transplantation. Drugs that can potentially induce or block a certain Tc subset are summarized in Table 2. In summary, exploring the potential of Tc subsets holds great promise for the advancement of innovative therapeutics in human diseases.
Concluding remarks
The diverse subsets of CD8 T cells, including Tc1 cells, Tc2 cells, Tc9 cells, Tc17 cells, Tc22 cells, Tfcs, and suppressive CD8 Tregs, have emerged as critical players in immune responses and disease pathogenesis. These subsets exhibit distinct phenotypic and functional characteristics, enabling them to fulfill specific roles in various immune contexts.
Importantly, these different subsets of CD8 T cells have been investigated in a wide range of disease contexts, including cancer, autoimmune disorders, and viral infections. The abundances, functions, and interactions within the tumor microenvironment of the immune system have been associated with disease severity, treatment response, and patient prognosis. Therefore, these cells hold significant potential as biomarkers for disease prediction, prognosis, and therapeutic targeting.
The characterization of Tc subsets has greatly expanded our understanding of their intricate immune responses and roles in various diseases. Further research in these areas will continue to elucidate the complex interplay between CD8 T-cell subsets and their importance in health and disease, ultimately improving the quality of life and overall survival of individuals.
References
Lee, S. W., Lee, G. W., Kim, H. O. & Cho, J. H. Shaping heterogeneity of naive CD8(+) T cell pools. Immune Netw. 23, e2 (2023).
Townsend, A. R., Gotch, F. M. & Davey, J. Cytotoxic T cells recognize fragments of the influenza nucleoprotein. Cell 42, 457–467 (1985).
Yewdell, J. W., Bennink, J. R., Smith, G. L. & Moss, B. Influenza A virus nucleoprotein is a major target antigen for cross-reactive anti-influenza A virus cytotoxic T lymphocytes. Proc. Natl Acad. Sci. USA 82, 1785–1789 (1985).
Ennis, F. A., Martin, W. J. & Verbonitz, M. W. Hemagglutinin-specific cytotoxic T-cell response during influenza infection. J. Exp. Med. 146, 893–898 (1977).
Townsend, A. R. & Skehel, J. J. Influenza A specific cytotoxic T-cell clones that do not recognize viral glycoproteins. Nature 300, 655–657 (1982).
Biddison, W. E., Shearer, G. M. & Chang, T. W. Regulation of influenza virus-specific cytotoxic T cell responses by monoclonal antibody to a human T cell differentiation antigen. J. Immunol. 127, 2236–2240 (1981).
Martin, M. D. & Badovinac, V. P. Defining memory CD8 T cell. Front. Immunol. 9, 2692 (2018).
Zhang, N. & Bevan, M. J. CD8(+) T cells: foot soldiers of the immune system. Immunity 35, 161–168 (2011).
Wherry, E. J. & Ahmed, R. Memory CD8 T-cell differentiation during viral infection. J. Virol. 78, 5535–5545 (2004).
Choi, H., Kim, Y. & Jung, Y. W. The function of memory CD8 + T cells in immunotherapy for human diseases. Immune Netw. 23, e10 (2023).
McLane, L. M., Abdel-Hakeem, M. S. & Wherry, E. J. CD8 T cell exhaustion during chronic viral infection and cancer. Annu. Rev. Immunol. 37, 457–495 (2019).
Ando, M., Ito, M., Srirat, T., Kondo, T. & Yoshimura, A. Memory T cell, exhaustion, and tumor immunity. Immunol. Med. 43, 1–9 (2020).
Wherry, E. J. T cell exhaustion. Nat. Immunol. 12, 492–499 (2011).
St. Paul, M. & Ohashi, P. S. The roles of CD8 + T cell subsets in antitumor immunity. Trends Cell Biol. 30, 695–704 (2020).
Perica, K., Varela, J. C., Oelke, M. & Schneck, J. Adoptive T cell immunotherapy for cancer. Rambam Maimonides Med. J. 6, e0004 (2015).
Seif, M., Einsele, H. & Löffler, J. CAR T cells beyond cancer: hope for immunomodulatory therapy of infectious diseases. Front. Immunol. 10, 2711 (2019).
Aghajanian, H., Rurik, J. G. & Epstein, J. A. CAR-based therapies: opportunities for immuno-medicine beyond cancer. Nat. Metab. 4, 163–169 (2022).
Mittrücker, H. W., Visekruna, A. & Huber, M. Heterogeneity in the differentiation and function of CD8+ T cells. Arch. Immunol. Ther. Exp. (Warsz) 62, 449–458 (2014).
Tang, R. et al. Ferroptosis, necroptosis, and pyroptosis in anticancer immunity. J. Hematol. Oncol. 13, 110 (2020).
Ito, N. et al. Prognostic significance of T helper 1 and 2 and T cytotoxic 1 and 2 cells in patients with non-small cell lung cancer. Anticancer Res. 25, 2027–2031 (2005).
Faghih, Z., Rezaeifard, S., Safaei, A., Ghaderi, A. & Erfani, N. IL-17 and IL-4 producing CD8 + T cells in tumor draining lymph nodes of breast cancer patients: positive association with tumor progression. Iran J. Immunol. 10, 193–204 (2013).
Podhorecka, M., Dmoszynska, A., Rolinski, J. & Wasik, E. T type 1/type 2 subsets balance in B-cell chronic lymphocytic leukemia–the three-color flow cytometry analysis. Leuk. Res. 26, 657–660 (2002).
Sun, Y. et al. Characterization of PD-L1 protein expression and CD8(+) tumor-infiltrating lymphocyte density, and their associations with clinical outcome in small-cell lung cancer. Transl. Lung Cancer Res. 8, 748–759 (2019).
Brambilla, E. et al. Prognostic effect of tumor lymphocytic infiltration in resectable non-small-cell lung cancer. J. Clin. Oncol. 34, 1223–1230 (2016).
Wu, Y. et al. T lymphocyte cell: a pivotal player in lung cancer. Front. Immunol. 14, 1102778 (2023).
Fluxá, P. et al. High CD8(+) and absence of Foxp3(+) T lymphocytes infiltration in gallbladder tumors correlate with prolonged patients survival. BMC Cancer 18, 243 (2018).
Chraa, D., Naim, A., Olive, D. & Badou, A. T lymphocyte subsets in cancer immunity: friends or foes. J. Leukoc. Biol. 105, 243–255 (2019).
Maleki Vareki, S. High and low mutational burden tumors versus immunologically hot and cold tumors and response to immune checkpoint inhibitors. J. Immunother. Cancer 6, 157 (2018).
Zhang, J., Huang, D., Saw, P. E. & Song, E. Turning cold tumors hot: from molecular mechanisms to clinical applications. Trends Immunol. 43, 523–545 (2022).
Nicolet, B. P. et al. CD29 identifies IFN-γ-producing human CD8(+) T cells with an increased cytotoxic potential. Proc. Natl Acad. Sci. USA 117, 6686–6696 (2020).
Schmitz, J. E. et al. Control of viremia in simian immunodeficiency virus infection by CD8+ lymphocytes. Science 283, 857–860 (1999).
Griffin, D. E., Lin, W. H. & Pan, C. H. Measles virus, immune control, and persistence. FEMS Microbiol. Rev. 36, 649–662 (2012).
Snyder, C. M. Buffered memory: a hypothesis for the maintenance of functional, virus-specific CD8(+) T cells during cytomegalovirus infection. Immunol. Res. 51, 195–204 (2011).
Shoukry, N. H. et al. Memory CD8 + T cells are required for protection from persistent hepatitis C virus infection. J. Exp. Med. 197, 1645–1655 (2003).
Loyal, L. et al. SLAMF7 and IL-6R define distinct cytotoxic versus helper memory CD8 + T cells. Nat. Commun. 11, 6357 (2020).
Lanzavecchia, A. & Sallusto, F. Regulation of T cell immunity by dendritic cells. Cell 106, 263–266 (2001).
de Araújo-Souza, P. S. et al. Differential interferon-γ production by naive and memory-like CD8 T cells. J. Leukoc. Biol. 108, 1329–1337 (2020).
Seok, J. et al. A virtual memory CD8(+) T cell-originated subset causes alopecia areata through innate-like cytotoxicity. Nat Immunol, https://doi.org/10.1038/s41590-023-01547-5 (2023).
Viano, M. E. et al. Virtual memory CD8(+) T cells: origin and beyond. J. Interferon. Cytokine Res. 42, 624–642 (2022).
Liu, Q., Sun, Z. & Chen, L. Memory T cells: strategies for optimizing tumor immunotherapy. Protein Cell 11, 549–564 (2020).
McMaster, S. R., Wilson, J. J., Wang, H. & Kohlmeier, J. E. Airway-resident memory CD8 T cells provide antigen-specific protection against respiratory virus challenge through rapid IFN-γ production. J. Immunol. 195, 203–209 (2015).
Yang, J. et al. Allograft rejection mediated by memory T cells is resistant to regulation. Proc. Natl Acad. Sci. USA 104, 19954–19959 (2007).
Edwards, J. et al. CD103(+) tumor-resident CD8(+) T cells are associated with improved survival in immunotherapy-naïve melanoma patients and expand significantly during anti-PD-1 treatment. Clin. Cancer Res. 24, 3036–3045 (2018).
Manjarrez-Orduño, N. et al. Circulating T cell subpopulations correlate with immune responses at the tumor site and clinical response to PD1 inhibition in non-small cell lung cancer. Front. Immunol. 9, 1613 (2018).
Reboursiere, E. et al. Increased frequencies of circulating and tumor-resident Vδ1(+) T cells in patients with diffuse large B-cell lymphoma. Leuk. Lymphoma 59, 187–195 (2018).
Kim, K. et al. Single-cell transcriptome analysis reveals TOX as a promoting factor for T cell exhaustion and a predictor for anti-PD-1 responses in human cancer. Genome Med. 12, 22 (2020).
Philip, M. & Schietinger, A. CD8 + T cell differentiation and dysfunction in cancer. Nat. Rev. Immunol. 22, 209–223 (2022).
Seo, H. et al. TOX and TOX2 transcription factors cooperate with NR4A transcription factors to impose CD8(+) T cell exhaustion. Proc. Natl Acad. Sci. USA 116, 12410–12415 (2019).
Li, H. et al. Dysfunctional CD8 T cells form a proliferative, dynamically regulated compartment within human melanoma. Cell 176, 775–789.e718 (2019).
Seo, H. et al. BATF and IRF4 cooperate to counter exhaustion in tumor-infiltrating CAR T cells. Nat. Immunol. 22, 983–995 (2021).
Miller, B. C. et al. Subsets of exhausted CD8 + T cells differentially mediate tumor control and respond to checkpoint blockade. Nat. Immunol. 20, 326–336 (2019).
Guo, X. et al. Global characterization of T cells in non-small-cell lung cancer by single-cell sequencing. Nat. Med. 24, 978–985 (2018).
Taguchi, T. et al. Analysis of Th1 and Th2 cells in murine gut-associated tissues. Frequencies of CD4+ and CD8 + T cells that secrete IFN-gamma and IL-5. J. Immunol. 145, 68–77 (1990).
Coyle, A. J. et al. Virus-specific CD8+ cells can switch to interleukin 5 production and induce airway eosinophilia. J. Exp. Med. 181, 1229–1233 (1995).
Croft, M., Carter, L., Swain, S. L. & Dutton, R. W. Generation of polarized antigen-specific CD8 effector populations: reciprocal action of interleukin (IL)-4 and IL-12 in promoting type 2 versus type 1 cytokine profiles. J. Exp. Med. 180, 1715–1728 (1994).
Sad, S., Marcotte, R. & Mosmann, T. R. Cytokine-induced differentiation of precursor mouse CD8 + T cells into cytotoxic CD8 + T cells secreting Th1 or Th2 cytokines. Immunity 2, 271–279 (1995).
Hinks, T. S. C., Hoyle, R. D. & Gelfand, E. W. CD8(+) Tc2 cells: underappreciated contributors to severe asthma. Eur. Respir. Rev. 28, https://doi.org/10.1183/16000617.0092-2019 (2019).
Hilvering, B. et al. Synergistic activation of pro-inflammatory type-2 CD8(+) T lymphocytes by lipid mediators in severe eosinophilic asthma. Mucosal Immunol. 11, 1408–1419 (2018).
Chen, W. et al. The roles of type 2 cytotoxic T cells in inflammation, tissue remodeling, and prostaglandin (PG) D(2) production are attenuated by PGD(2) receptor 2 antagonism. J. Immunol. 206, 2714–2724 (2021).
Gelfand, E. W. & Hinks, T. S. C. Is there a role for type 2 CD8 + T cells in patients with steroid-resistant asthma? J. Allergy Clin. Immunol. 144, 648–650 (2019).
Jia, Y. et al. Steroidogenic enzyme Cyp11a1 regulates Type 2 CD8 + T cell skewing in allergic lung disease. Proc. Natl Acad. Sci. USA 110, 8152–8157 (2013).
Ning, F. et al. Hypoxia enhances CD8(+) T(C)2 cell-dependent airway hyperresponsiveness and inflammation through hypoxia-inducible factor 1α. J. Allergy Clin. Immunol. 143, 2026–2037.e2027 (2019).
Qiu, S., Duan, X., Geng, X., Xie, J. & Gao, H. Antigen-specific activities of CD8 + T cells in the nasal mucosa of patients with nasal allergy. Asian Pac. J. Allergy Immunol. 30, 107–113 (2012).
Glück, J., Rogala, B., Rogala, E. & Oleś, E. Allergen immunotherapy in intermittent allergic rhinitis reduces the intracellular expression of IL-4 by CD8 + T cells. Vaccine 26, 77–81 (2007).
Gardner, L. M., Thien, F. C., Douglass, J. A., Rolland, J. M. & O’Hehir, R. E. Induction of T ‘regulatory’ cells by standardized house dust mite immunotherapy: an increase in CD4 + CD25+ interleukin-10 + T cells expressing peripheral tissue trafficking markers. Clin. Exp. Allergy 34, 1209–1219 (2004).
Seneviratne, S. L. et al. Allergen-specific CD8(+) T cells and atopic disease. J. Clin. Investig. 110, 1283–1291 (2002).
Czarnowicki, T. et al. Severe atopic dermatitis is characterized by selective expansion of circulating TH2/TC2 and TH22/TC22, but not TH17/TC17, cells within the skin-homing T-cell population. J. Allergy Clin. Immunol. 136, 104–115.e107 (2015).
Cosmi, L. et al. CRTH2 is the most reliable marker for the detection of circulating human type 2 Th and type 2 T cytotoxic cells in health and disease. Eur. J. Immunol. 30, 2972–2979 (2000).
Alcain, J. et al. Mechanisms of unconventional CD8 Tc2 lymphocyte induction in allergic contact dermatitis: role of H3/H4 histamine receptors. Front. Immunol. 13, https://doi.org/10.3389/fimmu.2022.999852 (2022).
Alcain, J., Podaza, E., Gori, M. S., Salamone, G. & Vermeulen, M. Modulation of dendritic cell apoptosis and CD8(+) cytotoxicity by histamine: role of protein kinase C. Mediators Inflamm. 2017, 9402814 (2017).
Amaral, M. M. et al. Histamine improves antigen uptake and cross-presentation by dendritic cells1. J. Immunol. 179, 3425–3433 (2007).
Bangert, C. et al. Persistence of mature dendritic cells, T(H)2 A, and Tc2 cells characterize clinically resolved atopic dermatitis under IL-4Rα blockade. Sci. Immunol. 6, https://doi.org/10.1126/sciimmunol.abe2749 (2021).
Do, J.-S. et al. Committed memory effector type 2 cytotoxic T (Tc2) cells are ineffective in protective anti-tumor immunity. Immunol. Lett. 95, 77–84 (2004).
Sheu, B.-C. et al. Predominant Th2/Tc2 polarity of tumor-infiltrating lymphocytes in human cervical cancer1. J. Immunol. 167, 2972–2978 (2001).
Hartana, C. A. et al. Urothelial bladder cancer may suppress perforin expression in CD8 + T cells by an ICAM-1/TGFβ2 mediated pathway. PLoS One 13, e0200079 (2018).
Visekruna, A. et al. Tc9 cells, a new subset of CD8(+) T cells, support Th2-mediated airway inflammation. Eur. J. Immunol. 43, 606–618 (2013).
Wang, W., Cheng, Z. S., Chen, Y. F. & Lin, Y. H. Increased circulating IL-9-producing CD8 + T cells are associated with eosinophilia and high FeNO in allergic asthmatics. Exp. Ther. Med. 12, 4055–4060 (2016).
Ding, P. et al. IL-9-producing CD8(+) T cells represent a distinctive subset with different transcriptional characteristics from conventional CD8(+) T cells, and partially infiltrate breast tumors. Int. J. Biochem. Cell Biol. 115, 105576 (2019).
Lu, Y. et al. Tumor-specific IL-9-producing CD8 + Tc9 cells are superior effector than type-I cytotoxic Tc1 cells for adoptive immunotherapy of cancers. Proc. Natl Acad. Sci. USA 111, 2265–2270 (2014).
Ma, X. et al. Cholesterol negatively regulates IL-9-producing CD8(+) T cell differentiation and antitumor activity. J. Exp. Med. 215, 1555–1569 (2018).
Xiao, L. et al. IL-9/STAT3/fatty acid oxidation-mediated lipid peroxidation contributes to Tc9 cell longevity and enhanced antitumor activity. J. Clin. Investig. 132, https://doi.org/10.1172/JCI153247 (2022).
Lückel, C., Picard, F. S. R. & Huber, M. Tc17 biology and function: novel concepts. Eur. J. Immunol. 50, 1257–1267 (2020).
Chellappa, S. et al. CD8 + T cells that coexpress RORγt and T-bet are functionally impaired and expand in patients with distal bile duct cancer. J. Immunol. 198, 1729–1739 (2017).
Hamada, H. et al. Tc17, a unique subset of CD8 T cells that can protect against lethal influenza challenge. J. Immunol. 182, 3469–3481 (2009).
Res, P. C. M. et al. Overrepresentation of IL-17A and IL-22 producing CD8 T cells in lesional skin suggests their involvement in the pathogenesis of psoriasis. PLoS One 5, e14108 (2010).
Harrison, O. J. et al. Commensal-specific T cell plasticity promotes rapid tissue adaptation to injury. Science 363, https://doi.org/10.1126/science.aat6280 (2019).
Kim, B. S. et al. Type 17 immunity promotes the exhaustion of CD8(+) T cells in cancer. J. Immunother. Cancer 9, https://doi.org/10.1136/jitc-2021-002603 (2021).
Yen, H.-R. et al. Tc17 CD8 T cells: functional plasticity and subset diversity. J. Immunol. 183, 7161–7168 (2009).
Flores-Santibáñez, F. et al. In vitro-generated Tc17 cells present a memory phenotype and serve as a reservoir of Tc1 cells in vivo. Front. Immunol. 9, https://doi.org/10.3389/fimmu.2018.00209 (2018).
Liu, C.-H. et al. Adoptive transfer of IL-4 reprogrammed Tc17 cells elicits anti-tumour immunity through functional plasticity. Immunology 166, 310–326 (2022).
Arra, A. et al. The differentiation and plasticity of Tc17 cells are regulated by CTLA-4-mediated effects on STATs. Oncoimmunology 6, e1273300 (2017).
Arra, A., Lingel, H., Pierau, M. & Brunner-Weinzierl, M.C. PD-1 limits differentiation and plasticity of Tc17 cells. Front. Immunol. 14, https://doi.org/10.3389/fimmu.2023.1104730 (2023).
Zenobia, C. & Hajishengallis, G. Basic biology and role of interleukin-17 in immunity and inflammation. Periodontology2000 69, 142–159 (2015).
McGeachy, M. J., Cua, D. J. & Gaffen, S. L. The IL-17 family of cytokines in health and disease. Immunity 50, 892–906 (2019).
Tom, M. R. et al. Novel CD8 + T-cell subsets demonstrating plasticity in patients with inflammatory bowel disease. Inflamm. Bowel Dis. 22, 1596–1608 (2016).
Globig, A. M. et al. High-dimensional profiling reveals Tc17 cell enrichment in active Crohn’s disease and identifies a potentially targetable signature. Nat. Commun. 13, 3688 (2022).
Ha, H.-L. et al. IL-17 drives psoriatic inflammation via distinct, target cell-specific mechanisms. Proc. Natl Acad. Sci. 111, E3422–E3431 (2014).
Liu, J. et al. Single-cell RNA sequencing of psoriatic skin identifies pathogenic Tc17 cell subsets and reveals distinctions between CD8(+) T cells in autoimmunity and cancer. J. Allergy Clin. Immunol. 147, 2370–2380 (2021).
Teunissen, M. B. M. et al. The IL-17A-producing CD8 + T-cell population in psoriatic lesional skin comprises mucosa-associated invariant T cells and conventional T cells. J. Investig. Dermatol. 134, 2898–2907 (2014).
Nanjappa, S. G. et al. Antifungal Tc17 cells are durable and stable, persisting as long-lasting vaccine memory without plasticity towards IFNγ cells. PLoS Pathog 13, e1006356 (2017).
Kong, X., Sun, R., Chen, Y., Wei, H. & Tian, Z. γδT cells drive myeloid-derived suppressor cell–mediated CD8 + T cell exhaustion in hepatitis B virus–induced immunotolerance. J. Immunol. 193, 1645–1653 (2014).
Tao, J. et al. CD8(+) T cells exhaustion induced by myeloid-derived suppressor cells in myelodysplastic syndromes patients might be through TIM3/Gal-9 pathway. J. Cell Mol. Med. 24, 1046–1058 (2020).
St. Paul, M. et al. IL6 induces an IL22 + CD8 + T-cell subset with potent antitumor function. Cancer Immunol. Res. 8, 321–333 (2020).
Sabat, R., Ouyang, W. & Wolk, K. Therapeutic opportunities of the IL-22-IL-22R1 system. Nat. Rev. Drug Discov. 13, 21–38 (2014).
Liu, Y. et al. Interleukin-21 induces the differentiation of human Tc22 cells via phosphorylation of signal transducers and activators of transcription. Immunology 132, 540–548 (2011).
Zhang, S. et al. Increased Tc22 and Treg/CD8 ratio contribute to aggressive growth of transplant associated squamous cell carcinoma. PLOS One 8, e62154 (2013).
Oliveira, L. M. S. et al. Increased frequency of circulating Tc22/Th22 cells and polyfunctional CD38−T cells in HIV-exposed uninfected subjects. Sci. Rep. 5, 13883 (2015).
Cagan, E. et al. The age-dependent role of Th22, Tc22, and Tc17 cells in the severity of pneumonia in COVID-19 immunopathogenesis. Viral Immunol. 35, 318–327 (2022).
Lv, Y., Ricard, L., Gaugler, B., Huang, H. & Ye, Y. Biology and clinical relevance of follicular cytotoxic T cells. Front. Immunol. 13, 1036616 (2022).
Elzein, S. M., Zimmerer, J. M., Han, J. L., Ringwald, B. A. & Bumgardner, G. L. CXCR5(+)CD8(+) T cells: a review of their antibody regulatory functions and clinical correlations. J. Immunol. 206, 2775–2783 (2021).
Quigley, M. F., Gonzalez, V. D., Granath, A., Andersson, J. & Sandberg, J. K. CXCR5 + CCR7- CD8 T cells are early effector memory cells that infiltrate tonsil B cell follicles. Eur. J. Immunol. 37, 3352–3362 (2007).
Valentine, K. M. et al. CD8 follicular T cells promote B cell antibody class switch in autoimmune disease. J Immunol 201, 31–40 (2018).
Shen, J. et al. A subset of CXCR5(+)CD8(+) T cells in the germinal centers from human tonsils and lymph nodes help B cells produce immunoglobulins. Front. Immunol. 9, 2287 (2018).
Jiao, Y. M. et al. Dichotomous roles of programmed cell death 1 on HIV-specific CXCR5(+) and CXCR5(-) CD8(+) T cells during chronic HIV infection. Front. Immunol. 8, 1786 (2017).
Perdomo-Celis, F., Taborda, N. A. & Rugeles, M. T. Circulating CXCR5-expressing CD8 + T-cells are major producers of IL-21 and associate with limited HIV replication. J. Acquir. Immune Defic. Syndr. 78, 473–482 (2018).
Roider, J. et al. High-frequency, functional HIV-specific T-follicular helper and regulatory cells are present within germinal centers in children but not adults. Front. Immunol. 9, 1975 (2018).
Zimmerer, J. M. et al. Antibody-suppressor CXCR5(+) CD8(+) T cellular therapy ameliorates antibody-mediated rejection following kidney transplant in CCR5 KO mice. Am. J. Transplant. 22, 1550–1563 (2022).
Zimmerer, J. M. et al. Antibody-suppressor CD8 + T cells require CXCR5. Transplantation 103, 1809–1820 (2019).
Zimmerer, J. M., Pham, T. A., Sanders, V. M. & Bumgardner, G. L. CD8 + T cells negatively regulate IL-4-dependent, IgG1-dominant posttransplant alloantibody production. J. Immunol. 185, 7285–7292 (2010).
Martinez-Usatorre, A. et al. Enhanced phenotype definition for precision isolation of precursor exhausted tumor-infiltrating CD8 T cells. Front. Immunol. 11, 340 (2020).
Yang, Z. Z. et al. PD-1 expression defines two distinct T-cell sub-populations in follicular lymphoma that differentially impact patient survival. Blood Cancer J. 5, e281 (2015).
Brummelman, J. et al. High-dimensional single cell analysis identifies stem-like cytotoxic CD8(+) T cells infiltrating human tumors. J. Exp. Med. 215, 2520–2535 (2018).
Hofland, T. et al. Human CXCR5(+) PD-1(+) CD8 T cells in healthy individuals and patients with hematologic malignancies. Eur. J. Immunol. 51, 703–713 (2021).
de Goer de Herve, M. G., Abdoh, M., Jaafoura, S., Durali, D. & Taoufik, Y. Follicular CD4 T cells tutor CD8 early memory precursors: an initiatory journey to the frontier of B cell territory. iScience 20, 100–109 (2019).
Im, S. J. et al. Defining CD8 + T cells that provide the proliferative burst after PD-1 therapy. Nature 537, 417–421 (2016).
Im, S. J., Konieczny, B. T., Hudson, W. H., Masopust, D. & Ahmed, R. PD-1+ stemlike CD8 T cells are resident in lymphoid tissues during persistent LCMV infection. Proc. Natl Acad. Sci. USA 117, 4292–4299 (2020).
Yang, M. et al. CXCL13 shapes immunoactive tumor microenvironment and enhances the efficacy of PD-1 checkpoint blockade in high-grade serous ovarian cancer. J. Immunother. Cancer 9, https://doi.org/10.1136/jitc-2020-001136 (2021).
Bai, M. et al. CXCR5(+) CD8(+) T cells potently infiltrate pancreatic tumors and present high functionality. Exp. Cell Res. 361, 39–45 (2017).
Huang, Q. et al. Identification and validation of an excellent prognosis subtype of muscle-invasive bladder cancer patients with intratumoral CXCR5(+) CD8(+) T cell abundance. Oncoimmunology 9, 1810489 (2020).
E, J. et al. CD8(+)CXCR5(+) T cells in tumor-draining lymph nodes are highly activated and predict better prognosis in colorectal cancer. Hum. Immunol. 79, 446–452 (2018).
Chu, F. et al. CXCR5(+)CD8(+) T cells are a distinct functional subset with an antitumor activity. Leukemia 33, 2640–2653 (2019).
Wang, J. et al. Intratumoral CXCR5(+)CD8(+)T associates with favorable clinical outcomes and immunogenic contexture in gastric cancer. Nat. Commun. 12, 3080 (2021).
Mishra, S., Srinivasan, S., Ma, C. & Zhang, N. CD8(+) regulatory T cell - a mystery to be revealed. Front. Immunol. 12, 708874 (2021).
Jiang, H., Zhang, S. I. & Pernis, B. Role of CD8 + T cells in murine experimental allergic encephalomyelitis. Science 256, 1213–1215 (1992).
Koh, D. R. et al. Less mortality but more relapses in experimental allergic encephalomyelitis in CD8-/- mice. Science 256, 1210–1213 (1992).
Noble, A., Zhao, Z. S. & Cantor, H. Suppression of immune responses by CD8 cells. II. Qa-1 on activated B cells stimulates CD8 cell suppression of T helper 2 responses. J. Immunol. 160, 566–571 (1998).
Kim, H. J. et al. CD8 + T regulatory cells express the Ly49 Class I MHC receptor and are defective in autoimmune prone B6-Yaa mice. Proc. Natl Acad. Sci. USA 108, 2010–2015 (2011).
Kim, H. J., Verbinnen, B., Tang, X., Lu, L. & Cantor, H. Inhibition of follicular T-helper cells by CD8(+) regulatory T cells is essential for self tolerance. Nature 467, 328–332 (2010).
Kim, H. J. & Cantor, H. Regulation of self-tolerance by Qa-1-restricted CD8(+) regulatory T cells. Semin. Immunol. 23, 446–452 (2011).
Kim, H. J. et al. Stable inhibitory activity of regulatory T cells requires the transcription factor Helios. Science 350, 334–339 (2015).
Mishra, S. et al. TGF-beta and Eomes control the homeostasis of CD8+ regulatory T cells. J. Exp. Med. 218, https://doi.org/10.1084/jem.20200030 (2021).
Jiang, H. et al. Regulatory CD8 + T cells fine-tune the myelin basic protein-reactive T cell receptor V beta repertoire during experimental autoimmune encephalomyelitis. Proc. Natl Acad. Sci. USA 100, 8378–8383 (2003).
Hu, D. et al. Analysis of regulatory CD8 T cells in Qa-1-deficient mice. Nat. Immunol. 5, 516–523 (2004).
Correale, J. & Villa, A. Isolation and characterization of CD8+ regulatory T cells in multiple sclerosis. J. Neuroimmunol. 195, 121–134 (2008).
Lu, L., Kim, H. J., Werneck, M. B. & Cantor, H. Regulation of CD8+ regulatory T cells: Interruption of the NKG2A-Qa-1 interaction allows robust suppressive activity and resolution of autoimmune disease. Proc. Natl Acad. Sci. USA 105, 19420–19425 (2008).
Saligrama, N. et al. Opposing T cell responses in experimental autoimmune encephalomyelitis. Nature 572, 481–487 (2019).
Kusnadi, A. et al. Severely ill COVID-19 patients display impaired exhaustion features in SARS-CoV-2-reactive CD8(+) T cells. Sci. Immunol. 6, https://doi.org/10.1126/sciimmunol.abe4782 (2021).
Li, J. et al. KIR(+)CD8(+) T cells suppress pathogenic T cells and are active in autoimmune diseases and COVID-19. Science 376, eabi9591 (2022).
Koh, J. Y., Kim, D. U., Moon, B. H. & Shin, E. C. Human CD8(+) T-cell populations that express natural killer receptors. Immune Netw. 23, e8 (2023).
Agle, K. et al. Bim regulates the survival and suppressive capability of CD8(+) FOXP3(+) regulatory T cells during murine GVHD. Blood 132, 435–447 (2018).
Beres, A. J. et al. CD8+ Foxp3+ regulatory T cells are induced during graft-versus-host disease and mitigate disease severity. J. Immunol. 189, 464–474 (2012).
Robb, R. J. et al. Identification and expansion of highly suppressive CD8(+)FoxP3(+) regulatory T cells after experimental allogeneic bone marrow transplantation. Blood 119, 5898–5908 (2012).
Sun, J. et al. Efficient therapeutic function and mechanisms of human polyclonal CD8(+)CD103(+)Foxp3(+) regulatory T cells on collagen-induced arthritis in mice. J. Immunol. Res. 2019, 8575407 (2019).
Zhong, H. et al. TGF-β-induced CD8(+)CD103(+) regulatory T cells show potent therapeutic effect on chronic graft-versus-host disease lupus by suppressing B cells. Front. Immunol. 9, 35 (2018).
Robert, C. et al. Pembrolizumab versus ipilimumab in advanced melanoma. N. Engl. J. Med. 372, 2521–2532 (2015).
Larkin, J. et al. Overall survival in patients with advanced melanoma who received nivolumab versus investigator’s choice chemotherapy in CheckMate 037: a randomized, controlled, open-label phase III trial. J. Clin. Oncol. 36, 383–390 (2018).
Migden, M. R. et al. PD-1 blockade with cemiplimab in advanced cutaneous squamous-cell carcinoma. N. Engl. J. Med. 379, 341–351 (2018).
Balar, A. V. et al. Atezolizumab (atezo) in first-line cisplatin-ineligible or platinum-treated locally advanced or metastatic urothelial cancer (mUC): long-term efficacy from phase 2 study IMvigor210. J. Clin. Oncol. 36, 4523–4523 (2018).
Antonia, S. J. et al. Durvalumab after chemoradiotherapy in stage III non-small-cell lung cancer. N. Engl. J. Med. 377, 1919–1929 (2017).
D’Angelo, S. P. et al. Avelumab in patients with previously treated metastatic Merkel cell carcinoma: long-term data and biomarker analyses from the single-arm phase 2 JAVELIN Merkel 200 trial. J. Immunother. Cancer 8, https://doi.org/10.1136/jitc-2020-000674 (2020).
Hodi, F. S. et al. Improved survival with ipilimumab in patients with metastatic melanoma. N. Engl. J. Med. 363, 711–723 (2010).
Munshi, N. C. et al. Idecabtagene vicleucel in relapsed and refractory multiple myeloma. N. Engl. J. Med. 384, 705–716 (2021).
Kamdar, M. et al. Lisocabtagene maraleucel versus standard of care with salvage chemotherapy followed by autologous stem cell transplantation as second-line treatment in patients with relapsed or refractory large B-cell lymphoma (TRANSFORM): results from an interim analysis of an open-label, randomised, phase 3 trial. Lancet 399, 2294–2308 (2022).
Berdeja, J. G. et al. Ciltacabtagene autoleucel, a B-cell maturation antigen-directed chimeric antigen receptor T-cell therapy in patients with relapsed or refractory multiple myeloma (CARTITUDE-1): a phase 1b/2 open-label study. Lancet 398, 314–324 (2021).
Maude, S. L. et al. Tisagenlecleucel in children and young adults with B-cell lymphoblastic leukemia. N. Engl. J. Med. 378, 439–448 (2018).
Wang, M. et al. KTE-X19 CAR T-cell therapy in relapsed or refractory mantle-cell lymphoma. N. Engl. J. Med. 382, 1331–1342 (2020).
Oluwole, O. O. et al. ZUMA-7: a phase 3 randomized trial of axicabtagene ciloleucel (Axi-Cel) versus standard-of-care (SOC) therapy in patients with relapsed/refractory diffuse large B cell lymphoma (R/R DLBCL). J. Clin. Oncol. 36, TPS7585–TPS7585 (2018).
Galon, J. & Bruni, D. Approaches to treat immune hot, altered and cold tumours with combination immunotherapies. Nat. Rev. Drug Discov. 18, 197–218 (2019).
Fabian, K. P., Padget, M. R., Fujii, R., Schlom, J. & Hodge, J. W. Differential combination immunotherapy requirements for inflamed (warm) tumors versus T cell excluded (cool) tumors: engage, expand, enable, and evolve. J. ImmunoTher. Cancer 9, e001691 (2021).
Gonem, S. et al. Fevipiprant, a prostaglandin D2 receptor 2 antagonist, in patients with persistent eosinophilic asthma: a single-centre, randomised, double-blind, parallel-group, placebo-controlled trial. Lancet Respir. Med. 4, 699–707 (2016).
Erpenbeck, V. J. et al. The oral CRTh2 antagonist QAW039 (fevipiprant): a phase II study in uncontrolled allergic asthma. Pulm Pharmacol. Ther. 39, 54–63 (2016).
Brightling, C. E. et al. Effectiveness of fevipiprant in reducing exacerbations in patients with severe asthma (LUSTER-1 and LUSTER-2): two phase 3 randomised controlled trials. Lancet Respir. Med. 9, 43–56 (2021).
Crotty Alexander, L. E. et al. Myeloid cell HIF-1α regulates asthma airway resistance and eosinophil function. J. Mol. Med. 91, 637–644 (2013).
Schedel, M. et al. 1,25D3 prevents CD8 + Tc2 skewing and asthma development through VDR binding changes to the Cyp11a1 promoter. Nat. Commun. 7, 10213 (2016).
Ogeyingbo, O. D. et al. The relationship between vitamin D and asthma exacerbation. Cureus 13, e17279 (2021).
Corren, J., Casale, T., Deniz, Y. & Ashby, M. Omalizumab, a recombinant humanized anti-IgE antibody, reduces asthma-related emergency room visits and hospitalizations in patients with allergic asthma. J. Allergy Clin. Immunol. 111, 87–90 (2003).
Noga, O. et al. Effect of omalizumab treatment on peripheral eosinophil and T-lymphocyte function in patients with allergic asthma. J. Allergy Clin. Immunol. 117, 1493–1499 (2006).
Cosgrove, S. B. et al. A blinded, randomized, placebo-controlled trial of the efficacy and safety of the Janus kinase inhibitor oclacitinib (Apoquel®) in client-owned dogs with atopic dermatitis. Vet. Dermatol. 24, e141–e582 (2013).
Jasiecka-Mikołajczyk, A., Jaroszewski, J. J. & Maślanka, T. Oclacitinib, a Janus kinase inhibitor, reduces the frequency of IL-4- and IL-10-, but not IFN-γ-, producing murine CD4(+) and CD8(+) T cells and counteracts the induction of type 1 regulatory T cells. Molecules 26, https://doi.org/10.3390/molecules26185655 (2021).
Yang, S. et al. TNF-a is a potent stimulator of Tc9-cell differentiation. J. Immunother. 43, 265–272 (2020).
Ramadan, A. et al. IL-33/ST2 triggering of IL-9-secreting T cells alters the balance of fatal immunity and tumor immunity. Blood 126, 231–231 (2015).
Solimani, F. et al. Therapeutic targeting of Th17/Tc17 cells leads to clinical improvement of Lichen Planus. Front. Immunol. 10, 1808 (2019).
Liu, P., Du, R. & Yu, X. Ursolic acid exhibits potent anticancer effects in human metastatic melanoma cancer cells (SK-MEL-24) via apoptosis induction, inhibition of cell migration and invasion, cell cycle arrest, and inhibition of mitogen-activated protein kinase (MAPK)/ERK signaling pathway. Med. Sci. Monit. 25, 1283–1290 (2019).
Qian, Z. et al. A phase I trial to evaluate the multiple-dose safety and antitumor activity of ursolic acid liposomes in subjects with advanced solid tumors. BioMed. Res. Int. 2015, 809714 (2015).
Lückel, C. et al. IL-17(+) CD8(+) T cell suppression by dimethyl fumarate associates with clinical response in multiple sclerosis. Nat. Commun. 10, 5722 (2019).
Klein Geltink, R. I. & Pillai, A. Executive CoAching unleashes Tc22 anti-tumor capacity. Sci. Immunol. 7, eabn9190 (2022).
St Paul, M. et al. Coenzyme A fuels T cell anti-tumor immunity. Cell Metab. 33, 2415–2427.e2416 (2021).
Flippe, L., Bézie, S., Anegon, I. & Guillonneau, C. Future prospects for CD8(+) regulatory T cells in immune tolerance. Immunol. Rev. 292, 209–224 (2019).
Guillonneau, C. et al. CD40Ig treatment results in allograft acceptance mediated by CD8CD45RC T cells, IFN-gamma, and indoleamine 2,3-dioxygenase. J. Clin. Investig. 117, 1096–1106 (2007).
Izawa, A. et al. A novel alloantigen-specific CD8 + PD1+ regulatory T cell induced by ICOS-B7h blockade in vivo. J. Immunol. 179, 786–796 (2007).
Picarda, E. et al. Transient antibody targeting of CD45RC induces transplant tolerance and potent antigen-specific regulatory T cells. JCI Insight 2, e90088 (2017).
Notley, C. A., McCann, F. E., Inglis, J. J. & Williams, R. O. ANTI-CD3 therapy expands the numbers of CD4+ and CD8+ Treg cells and induces sustained amelioration of collagen-induced arthritis. Arthritis Rheumatol. 62, 171–178 (2010).
Bisikirska, B., Colgan, J., Luban, J., Bluestone, J. A. & Herold, K. C. TCR stimulation with modified anti-CD3 mAb expands CD8 + T cell population and induces CD8 + CD25+ Tregs. J. Clin. Investig. 115, 2904–2913 (2005).
Horwitz, D. A., Bickerton, S., Koss, M., Fahmy, T. M. & La Cava, A. Suppression of murine Lupus by CD4+ and CD8+ Treg cells induced by T cell-targeted nanoparticles loaded with interleukin-2 and transforming growth factor β. Arthritis Rheumatol. 71, 632–640 (2019).
Bézie, S. et al. IL-34 is a Treg-specific cytokine and mediates transplant tolerance. J. Clin. Investig. 125, 3952–3964 (2015).
Bézie, S. et al. Human CD8+ Tregs expressing a MHC-specific CAR display enhanced suppression of human skin rejection and GVHD in NSG mice. Blood Adv. 3, 3522–3538 (2019).
Reiss, T. F. et al. Montelukast, a once-daily leukotriene receptor antagonist, in the treatment of chronic asthma: a multicenter, randomized, double-blind trial. Montelukast Clinical Research Study Group. Arch. Intern. Med. 158, 1213–1220 (1998).
Thurmond, R. L. et al. Clinical development of histamine H(4) receptor antagonists. Handb. Exp. Pharmacol. 241, 301–320 (2017).
Papp, K. A. et al. Efficacy and safety of ustekinumab, a human interleukin-12/23 monoclonal antibody, in patients with psoriasis: 52-week results from a randomised, double-blind, placebo-controlled trial (PHOENIX 2). Lancet. 371, 1675–1684 (2008).
Berger, T. et al. Effectiveness of delayed-release dimethyl fumarate on patient-reported outcomes and clinical measures in patients with relapsing-remitting multiple sclerosis in a real-world clinical setting: PROTEC. Mult. Scler. J. Exp. Transl. Clin. 5, 2055217319887191 (2019).
Acknowledgements
We thank all the Chung laboratory members for discussion and suggestions. Figures were created with BioRender.com. This work was supported by the research grants from the Leader Research Program (2020R1A3B207889011 to Y.C.), Basic Science Research Program (2022R1A6A1A03046247 to Y.C.), and Sejong Science Research Fellowship (2022R1C1C201061212 to C.H.K.) from National Research Foundation of Korea.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Koh, CH., Lee, S., Kwak, M. et al. CD8 T-cell subsets: heterogeneity, functions, and therapeutic potential. Exp Mol Med 55, 2287–2299 (2023). https://doi.org/10.1038/s12276-023-01105-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s12276-023-01105-x
This article is cited by
-
TRIM5 as a promising diagnostic biomarker of hepatocellular carcinoma: integrated analysis and experimental validation
Functional & Integrative Genomics (2024)