Abstract
The endoplasmic reticulum (ER) is a subcellular organelle essential for cellular homeostasis. Perturbation of ER functions due to various conditions can induce apoptosis. Chronic ER stress has been implicated in a wide range of diseases, including autosomal dominant retinitis pigmentosa (ADRP), which is characterized by age-dependent retinal degeneration caused by mutant rhodopsin alleles. However, the signaling pathways that mediate apoptosis in response to ER stress remain poorly understood. In this study, we performed an unbiased in vivo RNAi screen with a Drosophila ADRP model and found that Wg/Wnt1 mediated apoptosis. Subsequent transcriptome analysis revealed that ER stress-associated serine protease (Erasp), which has been predicted to show serine-type endopeptidase activity, was a downstream target of Wg/Wnt1 during ER stress. Furthermore, knocking down Erasp via RNAi suppressed apoptosis induced by mutant rhodopsin-1 (Rh-1P37H) toxicity, alleviating retinal degeneration in the Drosophila ADRP model. In contrast, overexpression of Erasp resulted in enhanced caspase activity in Drosophila S2 cells treated with apoptotic inducers and the stabilization of the initiator caspase Dronc (Death regulator Nedd2-like caspase) by stimulating DIAP1 (Drosophila inhibitor of apoptosis protein 1) degradation. These findings helped identify a novel cell death signaling pathway involved in retinal degeneration in an autosomal dominant retinitis pigmentosa model.
Similar content being viewed by others
Introduction
Signaling pathways related to stress responses help organisms survive under hostile conditions, either by protecting cells through damage repair and adaptation or by eliminating damaged cells through active cell death programs, such as apoptosis. The induction of cell death is beneficial for tissues in which lost cells can be readily replaced. In contrast, certain conditions that trigger the death of irreplaceable vital cells, including ER stress-induced cell death, underlie numerous neurodegenerative, digestive, and metabolic diseases in humans and model organisms1.
Stress responses of the endoplasmic reticulum (ER), triggered by the accumulation of unfolded proteins in the ER lumen, lead to the activation of multiple intracellular signaling pathways; this response is widely referred to as the unfolded protein response (UPR)2. Among known UPR pathways, a pathway regulated by IRE1 and XBP1 has been extensively characterized. IRE1 is a transmembrane ER protein with a luminal domain that can sense the presence of misfolded proteins3,4. The endonuclease domain in the cytoplasmic region of IRE1 catalyzes XBP1 mRNA splicing. The resultant frameshift in XBP1 mRNA translation generates an active isoform of XBP15,6,7. In addition to the IRE1/XBP1 pathway, two other well-characterized UPR pathways are regulated by ATF6 and PERK/ATF4. The critical functions of these pathways lead to the ultimate restoration of ER capacity by inducing the transcription of genes that encode ER chaperones, antioxidant proteins, and proteins involved in the degradation of misfolded proteins2.
Similar to cells under other stress conditions, certain cells are vulnerable to apoptosis under conditions of chronic ER stress. Consistently, the expression of a number of pro-apoptotic genes, including Bim, Noxa, and FasR, is induced in response to ER stress8,9,10, suggesting the activation of signaling pathways that promote cell death. Given that a better understanding of such pro-apoptotic pathways may pave the way for developing new approaches for treating ER stress-associated degenerative disease, models that can be used to elucidate the relevant pathways have been proposed. These models include a pro-apoptotic branch of signaling that originates from the IRE1 or ATF4 pathways11,12, proteolytic activation of caspases in response to abnormal Ca2+ levels13,14, and pathways that are initiated by excessive reactive oxygen species (ROS) that are generated in the stressed ER15,16,17. However, these models have no consensus, and their pathological significance still needs to be clarified.
The Wnt signaling pathway is a crucial regulator of embryonic development across species and governs homeostatic self-renewal in diverse adult tissues18,19,20. In addition to its roles in cell proliferation and differentiation during development, Wnt signaling regulates retinal development through apoptosis. Inactivation of a Drosophila homolog of the tumor suppressor adenomatous polyposis coli (APC) or Armadillo overexpression, which mediates canonical Wnt signaling, causes retinal degeneration via apoptosis. This phenotype is similar to that observed in humans with congenital hypertrophy of the retinal pigment epithelium (CHRPE)21. Moreover, Wnt1 signaling is indispensable for regulating peripheral ommatidial cell death during the pupal stage in Drosophila22,23. During eye development, Wnt-mediated apoptosis plays a crucial role in refining the ordered structure of the compound eye by eliminating excess ommatidial cells.
In this study, we report a novel function of Wg/Wnt1 in apoptosis caused by ER stress. We established a Drosophila model to study the molecular processes associated with ER stress-triggered cell death and performed an in vivo RNAi screen using this model. RNAi screening, followed by subsequent transcriptome sequencing studies, led to the identification of novel signaling mediators of ER stress-induced apoptosis. These findings were validated using classical gain-in-function and knockdown studies.
Materials and Methods
Fly stocks
All Drosophila melanogaster stocks were maintained with standard cornmeal medium containing 1.6% yeast, 0.9% soy flour, 6.7% cornmeal, 1% agar, and 7% light corn syrup at 25 °C. The expression of genes in Drosophila eyes is mediated through the Gal4/UAS system24. The following fly lines, which have been previously described, were used in this study: GMR-GAL425, UAS-DICER226, xbp1p > dsRed27, ninaEG69D28, and Rh1-GFP29. UAS-DIAP1 flies were obtained from FlyORF (http://flyorf.ch). The RNAi lines for the in vivo RNAi screen were obtained from the Vienna Drosophila Resource Center, Vienna, Austria (http://stockcenter.vdrc.at). Erasp mutant alleles were generated via the CRISPR–Cas9 system. Two gRNA sequences were used to target Cas9 to the Erasp coding region. The targeting gRNA sequences used against the coding region of Erasp were gRNA1: 5'-AAAACGATCGACTGTCAGCTCGG-3'; gRNA2: 5'-CATTTTGCGAGACGGTATCTTGG-3'.
Plasmid construction
The coding sequence of Rh-1P37H was amplified from Rh1 > ninaEP37H using RT‒PCR30 and subcloned into pUAST and pGMR vectors25. The GMR-Rh-1P37H line was used for the in vivo RNAi screen. HA-tagged Erasp, V5-tagged Erasp, HA-tagged DIAP1, DIAP1-EGFP, and Dronc-EGFP were subcloned into a pUAST vector. The Erasp mutant (S228A) was generated using a QuickChange site-directed mutagenesis kit (Stratagene, San Diego, CA, USA). The sequence of the mutant was verified by DNA sequencing.
Immunoblotting and immunoprecipitation
For immunoblotting, total proteins were extracted from Drosophila S2 cells with lysis buffer containing 10 mM Tris-HCl pH 7.5; 1 mM EDTA, pH 8.0; 150 mM NaCl; 1% SDS; and a protease inhibitor cocktail (Roche Diagnostics GmbH., Mannheim, Germany). For immunoblotting, eye imaginal discs were harvested from flies characterized by one of each genotype, and proteins were extracted with 2X Laemmli buffer. After centrifugation at 15,700 × g for 10 min at 4 °C, the proteins in the supernatants were resolved by SDS‒PAGE and transferred to polyvinylidene difluoride membranes (Merck Millipore, MA, USA). For the coimmunoprecipitation assay, cells were lysed in lysis buffer (containing 50 mM Tris-HCl, pH 8.0; 150 mM NaCl; 1% NP40; 1 mM DTT; and protease inhibitor; Roche) for 20 min and centrifuged at 15,700 × g for 10 min at 4 °C. The supernatants were used for subsequent immunoprecipitation. The immunoprecipitation of DIAP1-EGFP was performed with an anti-EGFP antibody and protein A agarose beads (Bio-Rad Laboratories, Inc., CA, USA). After the beads were washed three times with the same buffer, the immunoprecipitated proteins were analyzed via SDS‒PAGE/immunoblotting. The following antibodies were used in this study:
Antibody against | Source | 1st Antibody dilution | Secondary antibody | 2nd Antibody dilution |
|---|---|---|---|---|
4C5 | DSHB | 1:500 | Anti-Rabbit Alexa-488 Thermo Fisher Scientific | 1:500 |
ATF4 | 1:500 | Anti-guinea pig Alexa-488 Thermo Fisher Scientific | 1:500 | |
dsRed | Clontech 632496 | 1:500 | Anti-Rabbit Alexa-594 Thermo Fisher Scientific | 1:500 |
V5 | Invitrogen R960-25 | 1:6000 | Anti-mouse IgG-HRP Jackson ImmunoResearch | 1:5000 |
HA | Roche 3F10 | 1:10,000 | Anti-rat IgG-HRP Jackson ImmunoResearch | 1:5000 |
EGFP | Invitrogen 6455 | 1:5000 | Anti-Rabbit IgG-HRP Jackson ImmunoResearch | 1:5000 |
α-tubulin | MBL PM054 | 1:10,000 | Anti-Rabbit IgG-HRP Jackson ImmunoResearch | 1:5000 |
Cell culture and transient transfection
Drosophila S2 cells were cultured in Schneider’s Drosophila medium (Cat# 21720; Invitrogen, Thermo Fisher Scientific Corp., MA, USA) supplemented with 10% fetal bovine serum (Cat# 16000-044, Invitrogen) and 0.5% penicillin/streptomycin (Cat# 15140-122, Invitrogen). The indicated genes were cloned into a pUAST vector and transfected into cells using Effectene™ (QIAGEN GmbH Company, Hilden, Germany). Drosophila S2R+ cells were transfected with a pMK-GFP-Wg plasmid to induce Wg expression and subsequently stimulated by 500 μM CuSO4 treatment for the indicated times.
Immunohistochemistry
All fluorescence images were obtained with a Zeiss LSM710 confocal microscope using a 20X objective lens. The following antibodies were used: rabbit anti-cleaved caspase-3 (diluted 1:50; #9661, Cell Signaling, MA, USA), monoclonal anti-rhodopsin1 (diluted 1:500; Developmental Studies Hybridoma Bank, University of Iowa, IA, USA), rabbit anti-dsRed (diluted 1:500; Clontech Laboratories, Inc., CA, USA), and guinea pig anti-ATF4 (diluted 1:50032). In addition, Apoptosis was analyzed by TUNEL staining using an ApopTag Red In Situ Apoptosis Detection kit (S7165, Merck Millipore, MA, USA).
Analysis of retinal degeneration
As eye pigment can affect retinal degeneration, flies expressing RNA interference (RNAi) against the white-encoding gene (Bloomington stock center #33613, Bloomington, Indiana, IN, USA) were used to analyze retinal degeneration. The flies were reared in vials (20–30 flies in each vial) under permanent lighting conditions at 25 °C. The vials were changed every 2 days. The pseudopupils were quantified with samples placed on a pad under blue fluorescent light after anesthetizing the flies with CO2. Cross-sectioning was carried out as previously described33, and toluidine blue dye was used to increase the contrast.
Caspase activity assays
Caspase activity was determined as previously described34. Cycloheximide and actinomycin D were purchased from Sigma‒Aldrich, MO, USA (Cat# C7698 and A9415, respectively). Following the indicated treatment, Drosophila S2 cells were extracted in caspase assay buffer (50 mM HEPES, pH 7.5; 100 mM NaCl; 1 mM EDTA; 0.1% CHAPS; 10% sucrose; 0.5% Triton X-100; 4% glycerol; 5 mM DTT, and a protease inhibitor cocktail from Roche). Protein concentrations were determined using the Bradford assay. Homogenates (40 μg per assay) were incubated with 100 μM Ac-DEVD-AFC (Cat# ALX-260-032; Enzo Life Sciences, NY, USA) in a final volume of 100 μL of caspase buffer. Fluorescence was measured at excitation and emission wavelengths of 380 nm and 460 nm, respectively, and the reaction was monitored at 30 min intervals for 2 h at 37 °C with a Victor X4 2030 Multilabel Reader (PerkinElmer Inc., MA, USA).
RNA sequencing
Total RNA was isolated from flies using TRIzol reagent (Invitrogen). A cDNA library was prepared with 1 μg of total RNA from each sample using an Illumina TruSeq mRNA Sample Prep kit (Illumina Inc., CA, USA). The libraries were quantified via qPCRs according to the qPCR Quantification Protocol Guide (KAPA Library Quantification kits for Illumina Sequencing platforms) and qualified using a 2100 Bioanalyzer (Agilent Technologies, Waldbronn, Germany). Subsequently, indexed libraries were sequenced using the HiSeq4000 platform by Macrogen Inc., Seoul, Republic of Korea.
Read mapping and differential gene expression analysis
Reads were filtered with NGS QC Toolkit35 (v.2.3.3) and mapped to the D. melanogaster reference transcriptome assembly (dm6) with Bowtie36 and RSEM37. Differential gene expression analysis was done with expected count data from RSEM results using DEseq238. All the P-values were adjusted using multiple testing with a Benjamini‒Hochberg correction and a false discovery rate (FDR) of 5%. Differentially expressed genes were identified via the following thresholds: a fold change >2, a P-value < 0.05, and an adjusted P-value < 0.05.
Enrichment analysis
Identification of GO biological process terms and KEGG pathways related to genes that were significantly altered in the RNA-sequencing studies was performed using the Database for Annotation, Visualization, and Integrated Discovery (DAVID)39. GO biological process terms exhibiting significant enrichment were selected based on P-values < 0.05 and FDR < 0.05.
RT‒PCR and RT-quantitative PCR
Total RNA was isolated using TRIzol reagent (Invitrogen), and 100 ng of total RNA was transcribed with a ReverTra Ace qPCR RT Kit (Toyobo Co., Osaka, Japan). Quantitative PCR amplification was performed for 40 cycles using TOPreal™ qPCR 2X PreMIX (SYBR Green with high ROX) and a LightCycler® 480 Real-Time PCR System (Roche Diagnostics). Rp49 was the reference gene used for normalization. Relative quantification of mRNA was performed using the comparative CΤ method. PCR was performed using the following program with iProof™ High-Fidelity DNA polymerase (Cat#1725301; Bio-Rad) on a C1000 Touch™ Thermal Cycler (Bio-Rad): 98 °C for 30 s, 98 °C for 10 s, 57 °C for 30 s, 72 °C for 30 s (for a total of 28 cycles), and 72 °C for 10 min. The primer sequences are listed below:
Gene name | Forward sequences | Reverse sequences |
|---|---|---|
CG30090 | GTACAGATGTGGGGCGCTAT | CTCTCCATTGCCCAGAGAAC |
Z600 | TCGACAAATGAAACCAACCA | AACTCATGCTGCTCCTTTGG |
Lim1 | AATATCGGTCGCAGTGAACC | CAGGAACTTGTCCAGGATGG |
pirk | CGATTCGTATGACGATGACG | GCGTGGAACTTTTCTTGCTC |
CG34034 | AACGGGCACATTCAAACAAT | ATTTTGCTGCCGAATCAATC |
Unc-115b | GGGCAAGACCTATCACCAGA | CCGGTGTTGGTCACCTTACT |
CR40190 | CTGGGCGAAACTATTTCCAA | CCTCGACAGGAATCCGTTTA |
GstE9 | AGCGTTCATCGGTAATCAGG | CAATCCCACTAGGCTGGAGA |
GstE8 | ACTGCGTGGATCAAGAGGAT | TTGAGGAGGGTCACCAAATC |
CG11854 | AATGGCATTGCTGACATACG | CTCGGCGTAGGTTTGATGAT |
CG31676 | GTGATTTCGCGTTCCTGTTT | GCGGCAGTAGACGGTTTTTA |
rp49 | AGATCGTGAAGAAGCGCACCAAG | CACCAGGAACTTCTTGAATCCGG |
CG16974 | CTTAACTGCGAGCATGTGGA | CGTGGAAGCTCAGTCAACAA |
diap1 | TTGTGCAAGATCTGCTACGG | CCGCCCACATTTTCTTTTTA |
wg | CAACTTGGCCATTAGCGAGT | ATTTTTGCCCCTCGAGAAGT |
GST pulldown assay
The GST fusion protein, which was expressed in Escherichia coli strain BL21, was prepared in GST-binding buffer (50 mM Tris-HCl, pH 8.0; 150 mM NaCl; 0.1 mM EDTA and a protease inhibitor cocktail (Roche Diagnostics GmbH., Mannheim, Germany)) and purified with GSH-Sepharose beads (Amersham, UK). HA-tagged Erasp was expressed in S2 cells, extracted with lysis buffer containing 50 mM Tris-HCl, pH 8.0; 150 mM NaCl; 1% Triton X-100;0.1% SDS; and 0.5% sodium deoxycholate and incubated with purified GST fusion protein-bound beads for 4 h at 4 °C. The beads were washed extensively with the same lysis buffer to remove nonspecific proteins. The extracted proteins were boiled in 2X Laemmli buffer and subjected to SDS‒PAGE/immunoblotting.
Scanning electron microscopy
Standard procedures were used for sample preparation, including fixation in 2% glutaraldehyde, dehydration, and drying in CPD (HCP-2, HITACHI, Japan). Platinum was used to coat adult flies, and images were taken at 180 x and 500 x magnification.
Results
Wg/Wnt1 knockdown suppresses the Rh-1P37H overexpression-induced phenotype in Drosophila
In previous studies, we developed an in vivo model of ER stress-induced apoptosis in Drosophila that harbors a mutant rhodopsin-1 (Rh-1) allele, Rh-1G69D, and we identified several mediators of ER stress-induced apoptosis31,40. In this study, we modified the model to express the mutant Rh-1 allele Rh-1P37H, which is the equivalent of mammalian Rh-1P23H and the most common autosomal dominant retinitis pigmentosa (ADRP)-related mutation in humans41,42. Overexpression of the Rh-1P37H mutant in larval eye imaginal discs caused ER stress, as validated by three independent reporters (Supplementary Fig. 1), namely, xbp1p>dsRed27, ATF431, and XBP1-EGFP43. Moreover, Rh-1P37H-overexpressing flies presented with an abnormal external eye phenotype (Fig. 1a, b). This phenotype was suppressed by CDK5 knockdown (Supplementary Fig. 2), which was previously described as a mediator of ER stress-induced apoptosis31. All the detectable ER stress-related phenotypes were quite similar to those previously reported in Rh-1G69D mutant allele-harboring flies31.
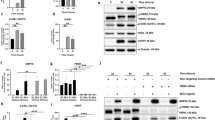
a–h External adult eyes misexpressing Rh-1P37H, together with genetic modifiers were identified through an in vivo RNAi screen. a Canton S. b Rh-1P37H-expressing flies without an inverted repeat transgene have abnormally small eyes with shinny surfaces. c–h Genetic modifiers. c Pirate, d Wg/Wnt1, e ISOT-3, f Lpt, g dPygo, and h CG12362. i, j The levels of arm/β-catenin in eye discs. The accumulation of arm/β-catenin due to Rh-1P37H misexpression was reduced in Wg/Wnt1 knocked down eye discs (i). Comparison of the relative expression of the arm in these eye imaginal discs (n = 5) (j). k, l Apoptosis of Rh-1P37H-misexpressing eye discs as assessed using TUNEL staining (red). Rh-1P37H-triggered apoptosis (k) was suppressed by the knockdown of Wg/Wnt1 (l). Green fluorescence indicates anti-Rh-1 staining. m Comparison of the numbers of apoptotic cells between those presented in (k) and (l) (n = 3). n, o Caspase activation due to misexpression of Rh-1P37H was suppressed under Wg/Wnt1 knockdown conditions. Larval eye imaginal discs were labeled with an anti-cleaved caspase antibody (white) to assess cell death. (p) Comparison of the numbers of active caspase-positive cells in (n) and (o) (n = 3). q, r The degree of ER stress was analyzed with the ER stress reporters xbp1p > dsRed and anti-ATF4. The transcriptional activation of XBP1 on misexpression of Rh-1P37H was assessed by dsRed expression (red). The degree of xbp1p > dsRed activation and ATF4 induction by Rh-1P37H misexpression was similar between (q) and (r). Green: ATF4, blue: rhodopsin-1. s Quantification of ATF4 induction by Rh-1P37H misexpression based on (Q” and R”) (n = 9). t The induction of dsRed was measured with the NIH ImageJ program. Wg/Wnt1 knockdown did not affect the degree of XBP1 pathway activation (n = 9). u The levels of Rh-1 were similar between (q) and (r) (n = 5). v Quantification of photoreceptor cell degeneration using a pseudopupil assay. For each genotype, the percentage indicates the number of flies with an intact Rh-1 > GFP pattern. Knockdown of Wg/Wnt1 suppressed the course of retinal degeneration in ninaEG69D/+ flies (n = 9). w, x Representative images of 32-day-old adult eye tangential sections with downregulated Wg/Wnt1 expression in the ninaEG69D/+ background. Error bars indicate ±S.E.M. P-values were determined using Student’s t-tests. *P < 0.05, **P < 0.01, and ***P < 0.001. The scale bar in (a) corresponds to 50 μm in (a–h) and 100 μm in (k) and (q). The scale bar in (w) corresponds to 5 μm (w, x).
Based on the external eye phenotype, a subsequent in vivo RNAi screen was conducted to identify genes that are critical for Rh-1P37H allele-associated toxicity. Specifically, we screened 354 inverted repeat transgenes that target ubiquitin-related genes (NYU-DRSC UBIQ, https://fgr.hms.harvard.edu/drsc-focused-sub-libraries) (Supplementary Table 1). Knockdown of the genes resulted in aggravation of the eye phenotype in most flies (Supplementary Fig. 3). In particular, our results demonstrated that six Drosophila lines showed profound suppression of adult eye phenotype (Fig. 1c–h), with two fly lines (VDRC104579 and VDRC100724) sharing the Wg/Wnt1 signaling pathway in common: one line (VDRC104579) targeted Drosophila Wg/Wnt1 and the other line (VDRC100724) targeted Pygopus, which is a key nuclear component of the Wnt signaling pathway. The other suppressors of Rh-1 toxicity were found to be Pirate, ISOT-3, Lpt, and CG12362 (Fig. 1c, e, f, and h).
Wg/Wnt1 is the main regulatory pathway in cell proliferation and differentiation during development and adult tissue homeostasis. In addition, aberrant Wnt signaling has often been attributed to multiple human diseases, including cancers19,44,45. Investigation of the knockdown efficiency of RNAi targeting Wg/Wnt1 as measured through quantitative RT‒PCR revealed that Wg/Wnt1 expression was knocked down by ~90% (Supplementary Fig. 4a). To verify the involvement of the Wg/Wnt1 signaling pathway in Rh-1P37H overexpression-induced ER stress, we examined the accumulation of armadillo/β-catenin, which is stabilized by Wnt activation20. The accumulation of armadillo/β-catenin on misexpression of Rh-1P37H was decreased when Wg/Wnt1 was knocked down (Fig. 1i, j), indicating that the Wg/Wnt1 signaling pathway is activated by ER stress. Subsequent loss-of-function studies revealed that Wg/Wnt1 knockdown itself did not affect the external eye phenotype (Supplementary Fig. 4b, c). In addition, Wg/Wnt1 knockdown did not influence the cell death phenotype induced by p53 overexpression (Supplementary Fig. 4d, e), indicating that Wg/Wnt1 mediates specific apoptotic signaling in response to mutant Rh-1 expression. However, examination of cell death in Rh-1P37H-expressing eye imaginal discs revealed that the proportion of TUNEL-positive cells was significantly reduced, by 70%, compared with that in control discs, indicating that Wg/Wnt1 is essential for apoptosis in Rh-1P37H-overexpressing eye imaginal discs (Fig. 1k–m). Furthermore, an investigation of cellular caspase activity revealed that active caspase staining was consistently reduced in Wg/Wnt1-knockdown eye discs (Fig. 1n–p).
To determine whether the suppression of apoptosis by Wg/Wnt1 knockdown is attributed to the reduction in overall ER stress, we used the ER stress reporter xbp1p > dsRed27 in eye imaginal discs misexpressing Rh-1P37H and labeled them with an anti-ATF4 antibody. Our results showed that the overall intensities of the xbp1p > dsRed reporter and the levels of induced ATF4, and Rh-1P37H expression upon misexpression Rh-1P37H were similar between the control and the Wg/Wnt1-knockdown groups (Fig. 1q–u). These results indicate that Wg/Wnt1 specifically mediates proapoptotic signaling without affecting the overall levels of ER stress. To test whether the Wg/Wnt1 pathway is relevant to age-dependent disease progression, we examined retinal degeneration in a Drosophila model of ADRP, in which a mutant allele of the Rh-1 gene, ninaEG69D, causes age-dependent retinal degeneration28,46. An Rh-1 > GFP reporter-based pseudopupil assay was performed to monitor retinal degeneration in live flies. The results demonstrated that the ninaEG69D/+ flies started to lose their pseudopupil at 12 d in a progressive manner, with only 20% of these flies presenting with an intact pseudopupil at 28 d after eclosion (Fig. 1v, blue line). However, the knockdown of Wg/Wnt1 in photoreceptor cells using the Rh-1 driver significantly delayed the time course of retinal degeneration, with 66% of the examined flies exhibiting intact Rh-1 > GFP patterns at 28 d (Fig. 1v, red line). The ommatidia in the retinas of 32-day-old ninaEG69D/+ flies were mostly disorganized (Fig. 1w), which was rescued by Wg/Wnt1 knockdown (Fig. 1x). Consistently, the knockdown of dPygo identified with Wg/Wnt1 from in vivo RNAi screen as a modulator of Rh-1P37H toxicity (Fig. 1g) suppressed apoptosis triggered by Rh-1P37H overexpression (Supplementary Fig. 5a–d).
To investigate whether the IRE1 pathway contributes to Wg/Wnt1-mediated apoptosis, we examined the degree of cell death by analyzing the adult eye phenotype. Knocking down ire1 using RNAi aggravated the eye phenotype caused by Rh-1P37H misexpression. However, the eye phenotype was largely restored when ire1 and Wg/Wnt1 were knocked down together (Supplementary Fig. 6). These results indicate that the IRE1 pathway protects against misfolded protein accumulation in the ER rather than simply activating proapoptotic signaling proteins. Altogether, these results indicate that the Wg/Wnt1 signaling pathway mediates the apoptosis of retinal cells under ER stress conditions and plays an essential role in the progression of retinal degeneration in the presence of ER stress.
RNA sequencing reveals the association of multiple transcription targets of Wg/Wnt1 in ER stress-mediated apoptosis of retinal cells in the Drosophila ADRP model
To identify the signaling pathway mediated by Wg/Wnt1 under ER stress, we analyzed the differentially expressed genes (DEGs) that were identified from the RNA-sequencing data of eye imaginal discs with the following genotypes: GMR > DICER2 (red), GMR > Wg/Wnt1 RNAi, DICER2 (green), GMR > Rh-1P37H, DICER2 (blue), and GMR > Rh-1P37H, Wg/Wnt1 RNAi, DICER2 (purple) (Fig. 2a). The analysis identified 1134 transcripts that were differentially expressed in all samples (Supplementary Table 2, P < 0.05), and only the transcripts with a fold change >2 were selected for the subsequent analysis. When we compared the gene expression pattern between control discs (GMR > DICER2) and Rh-1P37H-misexpressing imaginal discs (GMR > Rh-1P37H, DICER2), a total of 764 genes were shown to be differentially expressed. Of these DEGs, 402 and 362 genes were up- and downregulated upon Rh-1P37H misexpression, respectively (Fig. 2b and Supplementary Table 3). A subsequent Gene Ontology (GO) analysis categorized these DEGs into enriched functional groups, which included genes encoding proteins for axon guidance, development, and protein ubiquitination (Supplementary Fig. 7).
a Heatmap of the two-way hierarchical clustering showing five differentially expressed genes (fold change > 2, P < 0.05) in GMR > DICER2 (red, n = 3), GMR > Wg/Wnt1 RNAi, DICER2 (green, n = 3), GMR > Rh-1P37H, DICER2 (blue, n = 3), and GMR > Rh-1P37H, Wg/Wnt1 RNAi, DICER2 flies (purple, n = 3). b The number of upregulated (yellow) or downregulated (blue) genes in flies with the indicated genotypes (fold change > 2, P < 0.05). c Quantile-normalized values of select genes that were regulated by Wg/Wnt1 under ER stress in the indicated genotypes according to RNA-seq analysis (refer to Supplemental Table 4). d Quantitative RT‒PCR analysis of selected genes in eye imaginal discs with the indicated genotypes; the levels were normalized to those of rp49 and presented relative to those of GMR > DICER2. e Normalized Erasp mRNA expression after GFP-wg overexpression in eye imaginal discs. f ER stress induced in response to independent treatment with chemicals, such as DTT (1 mM), Tu (1 µg/mL), and Tg (1 µM), triggered the induction of Erasp at the transcript level in Drosophila S2R+ cells, whereas this induction of Erasp by Tg (1 μM) or Tu (1 µg/mL) was absent in Drosophila S2 cells (g). P-values were determined using Student’s t-tests. *P < 0.05, **P < 0.01, and ***P < 0.001.
To identify the transcriptional targets of Wg/Wnt1 during ER stress, we compared the gene expression between two samples, GMR > Rh-1P37H, Wg/Wnt1 RNAi, DICER2 and GMR > Rh-1P37H, DICER2. The analysis revealed that Wg/Wnt1 knockdown resulted in the down- and upregulation of 19 and 37 transcripts, respectively, compared with control fly lines expressing endogenous levels of Wg/Wnt1 (Fig. 2b and Supplementary Table 4). Consistent with the RNA-sequencing analysis data (Fig. 2a, c), the qPCR data showed that the expression of the genes GstE8, Unc-115b, CG11854, CG31676, and CG30090 was regulated by Wg/Wnt1 signaling (Fig. 2d). In addition, we found that all the genes we investigated were upregulated by ER stress, i.e., upon misexpression of Rh-1P37H compared with their expression in control wild-type flies. Given that CG30090 is the most highly ranked gene identified in the RNA sequencing studies and that its cellular function has not been fully characterized, we decided to further explore the novel functions of CG30090 in ER stress-mediated cell death in Drosophila.
To verify that the gene expression of CG30090 is regulated by Wg/Wnt1 in vivo, we misexpressed GFP-wg in eye discs and found that the CG30090 mRNA expression level was significantly increased by Wg/Wnt1 induction (Fig. 2e). To evaluate whether the expression of CG30090 is regulated by ER stress, we exposed Drosophila S2R+ cells—which express a Wg receptor, Drosophila frizzled 2 (Dfz2)47—to ER stress-inducing chemicals, namely, dithiothreitol (DTT), tunicamycin (Tu), and thapsigargin (Tg). As shown in Fig. 2f, the expression of CG30090 at the transcript level was increased in response to ER stress. However, thapsigargin and tunicamycin failed to induce the expression of CG30090 in Drosophila S2 cells—which do not express Dfz2— (Fig. 2g), indicating that the expression of CG30090 in response to ER stress is regulated by Wg/Wnt1 signaling. As CG30090 expression was identified under ER stress in the present study, we termed this gene ER stress-associated serine protease (Erasp) hereafter.
Erasp is required for ER stress-induced apoptosis of retinal cells in the Drosophila ADRP model
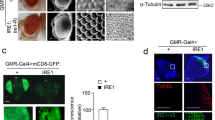
Wnt signaling pathways are divided into two types: canonical and noncanonical pathways20. The involvement of β-catenin characterizes the canonical pathway, whereas the noncanonical pathway functions independently of β-catenin. In the canonical pathway, upon Wnt activation, β-catenin is stabilized and transported from the cytosol to the nucleus, where it recruits TCF/LEF family cofactors to regulate the expression of target genes that are related to cell proliferation and differentiation. As we identified Erasp as a potential transcriptional target of Wg/Wnt1, we investigated whether the induction of Erasp is mediated by the canonical or noncanonical Wg/Wnt1 pathway. To this end, we induced the expression of Wg using the copper-inducible metallothionein promoter (pMT) in Drosophila S2R+ cells. Subsequent studies revealed that the expression of Erasp was increased by the overexpression of Wg. However, this increase in expression was abolished when the armadillo/β-catenin expression was knocked down using dsRNA targeting armadillo in Drosophila S2R+ cells, indicating that the induction of Erasp expression by Wg/Wnt1 activation is mediated through the canonical pathway (Fig. 3a, b). Furthermore, the noncanonical Wg/Wnt1 signaling pathway was also affected by ER stress, as the Rh-1P37H misexpression-induced activation of JNK, which is a mediator of the noncanonical Wnt signaling pathway, was suppressed when the Wg/Wnt1 level was decreased (Supplementary Fig. 8).
a The induction of Arm protein expression by GFP-Wg. Drosophila S2R+ cells transfected with pMK-GFP-Wg were incubated with CuSO4 for the indicated times. Lanes 1-6: control S2R+ cells, Lanes 7-12: S2R+ cells pretreated with dsRNA targeting arm. b The mRNA expression of Erasp after overexpression of Wg was analyzed in cells pretreated without or with dsRNA targeting arm. c–e Apoptosis in eye discs as assessed through TUNEL staining (red). Substantial apoptosis caused by Rh-1P37H misexpression (c') was significantly suppressed in Erasp knockdown in eye discs (d'). e The number of apoptotic cells in representative images was quantified (n = 9). Error bars indicate ± S.E.M. f Knockdown efficiency of Erasp RNAi in eye discs. Quantitative RT‒PCR results based on an analysis of dissected third-instar larval eye discs are shown. RNAi was expressed in tissues with the gmr-gal4 driver. The error bars indicate ± S.E.M. g, h The levels of apoptosis were assessed by labeling active caspase-3 in an Erasp-/- background. Misexpression of Rh-1P37H led to substantial apoptosis (g), which was suppressed in the Erasp-/- mutant (h). i, j External adult eyes. The degree of eye ablation as a result of Rh-1P37H misexpression (i) was suppressed in an Erasp-knockdown fly (j). k Quantification of age-related retinal degeneration in ninaEG69D/+ flies. Knockdown of Erasp suppressed retinal degeneration in this model (n = 7). l The overexpression of Erasp enhanced late-onset retinal degeneration of ninaEG69D/+ (n = 8). P-values were determined using Student’s t-tests. *P < 0.05, **P < 0.01, and ***P < 0.001. The scale bar in (c) represents 100 μm for (c) and (d) and that in (g) represents 50 μm for (g) and (h).
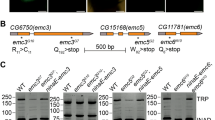
We further investigated whether Erasp plays a role in cell death caused by Rh-1P37H-associated toxicity. Consistent with the results obtained with Wg/Wnt1-knockdown Drosophila lines, knocking down Erasp in Drosophila eye imaginal discs suppressed the apoptosis that had been triggered by Rh-1P37H misexpression (Fig. 3c–e), even when the knockdown efficiency of Erasp RNAi was ~35% (Fig. 3f). This effect was verified using CRISPR‒Cas9-engineered Erasp-mutant flies (Supplementary Fig. 9). The Drosophila Erasp mutants were viable and exhibited no developmental defects. Accordingly, the substantial apoptosis induced by Rh-1P37H misexpression, measured by cleaved caspase-3 antibody labeling, was significantly reduced in flies with an Erasp-/- background (Fig. 3g, h). Moreover, the Rh-1P37H misexpression phenotype was suppressed in an Erasp-knockdown fly line (Fig. 3i, j). To investigate whether Erasp affects disease progression in a Drosophila model of ADRP, we performed a pseudopupil assay and found that knockdown of Erasp in photoreceptors delayed age-related retinal degeneration in the Drosophila model of ADRP (Fig. 3k). In contrast, overexpression of Erasp enhanced photoreceptor cell degeneration in the ADRP model (Fig. 3l). These results indicate that Erasp is involved in Rh-1P37H-induced apoptosis of retinal cells in the Drosophila model of ADRP.
Erasp mediates caspase-dependent apoptosis
To verify the apoptotic effect of Erasp, we examined caspase activity in Drosophila S2 cells overexpressing Erasp. Accordingly, we treated these cells with the apoptosis inducers cycloheximide (CHX) and actinomycin D. Further analysis revealed that stimulation of HA-tagged Erasp-overexpressing cells with CHX for 4 h significantly increased their caspase activity compared with that in control cells (EGFP-overexpressing cells) (Fig. 4a, c). Similarly, we performed fluorometric assays to measure caspase activity in Erasp-overexpressing cells, and the results revealed significantly higher cleavage of a synthetic DEVD substrate than that in control cells after treatment with actinomycin D for 4 h (Fig. 4b, c); these results indicated that Erasp mediates apoptosis via the activation of caspases.
a–c In vitro caspase activity was measured in control (EGFP-overexpressing S2 cells) or Erasp-HA-overexpressing cells. Cell death was triggered by CHX (a) or Act. D (b). The overexpression of Erasp enhanced the caspase activity induced by CHX or Act. D. c The control blot for (a) and (b). The expression of Erasp was measured with an anti-HA antibody. EGFP was used as a control gene. d The levels of Dronc were increased in an Erasp-HA dose-dependent manner. The amounts of Dronc fused to EGFP were measured by anti-EGFP antibody. α-Tubulin was used as a loading control. e The normalized band intensities of the gels shown in (d) were quantified, and the data are shown in the graph. f The levels of Dronc in eye imaginal discs. The level of Dronc, as measured with an anti-EGFP antibody, was significantly increased by the overexpression of Erasp in vivo. The graph indicates the relative levels of Dronc-EGFP (n = 3). g–j Representative eye phenotypes of flies expressing the indicated genes. The expression of genes was driven by the GMR-GAL4 driver. Scanning EM image of an external adult eye with Erasp misexpression shows a rough eye phenotype, missing bristles, and abnormal bristle production (h, h') compared to those in control (g, g'). The abnormal eye phenotype of Dronc-expressing flies (i) was aggravated by the coexpression of Erasp (j). P-values were determined using Student’s t-tests. *P < 0.05. The scale bar in (g) represents 60 μm for (g) and (h) and that in (g') represents 20 μm for (g') and (h').
In Drosophila, apoptotic stimuli induce Apaf-1 and the initiator caspase Dronc to form an apoptosome complex, which ultimately cleaves Dronc48,49,50. As Erasp is a serine protease with a trypsin domain, we examined the cleavage products of Dronc in the presence of Erasp to determine whether Erasp is involved in the proteolytic cleavage of Dronc. Our results showed that the levels of cleaved Dronc fragments were not significantly different, even in the presence of high concentrations of Erasp, compared with those under control conditions without Erasp (Fig. 4d). In contrast, we found that the levels of Dronc, as determined by the detection of the fusion protein—Dronc-EGFP—using an anti-EGFP antibody, gradually increased as the amount of Erasp was increased (Fig. 4d, e), indicating that Erasp was potentially involved in the stabilization of Dronc. To further validate these findings, we examined an in vivo model for the overexpression of Erasp using the eye-specific gene expression driver GMR-GAL4. Coexpression of Dronc-EGFP with Flag-tagged Erasp significantly increased the stability of Dronc compared to the control without Erasp expression (Fig. 4f). In addition, our analysis revealed that overexpressing Erasp resulted in a slightly rough eye phenotype, missing bristles, and abnormal bristle production, suggesting that an increased amount of Erasp alone can trigger apoptosis in vivo (Fig. 4g, g', h and h'). The combined expression of Dronc and HA-tagged Erasp further promoted the apoptosis of retinal cells, resulting in a severe rough eye phenotype, pigment loss, and eye abnormalities compared with those in Drosophila lines expressing Dronc alone (Fig. 4i, j). Altogether, these results indicate that Erasp is a novel mediator of the apoptosis pathway.
Erasp controls apoptosis by degrading DIAP1
Drosophila inhibitor of apoptosis protein 1 (DIAP1) is a key component of the apoptosis in Drosophila that suppresses the activation of caspases51,52,53. DIAP1 uses the E3 ubiquitin ligase activity of its RING domain to ubiquitylate Dronc directly, and it is autoubiquitylated in response to proapoptotic stimuli54,55,56. It has been reported that Drosophila OMI/HTRA2, which is a serine protease that has been characterized as an IAP antagonist, degrades DIAP157,58, thereby triggering apoptosis. Since Dronc was found to be stabilized by Erasp, we investigated whether Erasp is also involved in the degradation of DIAP1, similar to the effect of OMI/HTRA2. Accordingly, HA-tagged DIAP1 was cotransfected with Erasp-HA into Drosophila S2 cells, and subsequent studies revealed that DIAP1 expression was downregulated when coexpressed with Erasp in a dose-dependent manner (Fig. 5a, b). Next, we examined the interaction between Erasp and DIAP1 using coimmunoprecipitation assay. Our results demonstrated an evident interaction between DIAP1 and Erasp when DIAP1 fused with EGFP was coexpressed with HA-tagged Erasp in Drosophila S2 cells (Fig. 5c); notably, Erasp alone did not interact with EGFP (Fig. 5d).
a Drosophila S2 cells were cotransfected with Erasp-HA and DIAP1-HA. Whole-cell lysates were immunoblotted to determine DIAP1 and Erasp expression levels. b Graph showing quantified and normalized band intensities from the immunoblot (a). Error bars show ± S.E.M. c Erasp physically interacts with DIAP1 in Drosophila S2 cells. DIAP1 was immunoprecipitated with an anti-EGFP antibody, and its interaction partner, Erasp, was detected through its HA epitope. d EGFP does not interact with Erasp. An asterisk indicates the light chain of the antibody. WCL: Whole-cell lysate. e DIAP1 degradation was accelerated by the overexpression of Erasp. HA-tagged Erasp and V5-tagged DIAP1 were transiently cotransfected into S2 cells. Then, chase experiments were performed at the indicated time points after the addition of 1 μg/mL cycloheximide (CHX) at time zero. α-Tub was used as a loading control. f Quantification of DIAP1-V5 signals in (e) normalized to those of endogenous α-tubulin. g Graphs show the rate of DIAP1 degradation. The DIAP1 level at 0 h chase in each panel of (e) was set to 1. h GST pulldown assay. GST-DIAP1 and truncation mutants purified using GST-Sepharose beads were incubated with HA-tagged Erasp-overexpressing S2R+ cells for 4 h at 4 °C. The bead complexes were separated by SDS‒PAGE and immunoblotted using an anti-HA antibody. i Compared to the effect of wild-type Erasp (EraspWT-HA) on DIAP1 stability, catalytically inactive Erasp (EraspS228A-HA) restored the levels of DIAP1. The graphs show the normalized band intensities from the blot. j–l External adult eye phenotypes caused by Rh-1P37H overexpression, together with the indicated genes. m–o Scanning EM image of external adult eyes. The expression of Rh-1P37H in larval eye discs resulted in small adult eyes with abnormally smooth surfaces (m), and this effect was partially reversed by DIAP1 coexpression (n). The eye phenotype rescued by DIAP1 expression was aggravated by Erasp coexpression (o). The scale bar in (m) represents 60 μm for (m–o). Error bars indicate ± S.E.M. P-values were determined using Student’s t-tests. *P < 0.05, **P < 0.01, and *** P < 0.001.
To further determine that Erasp degrades DIAP1, we performed a chase experiment using cycloheximide (CHX), which inhibits protein synthesis by interfering with the translocation step during translation. Consequently, we observed that the levels of V5-tagged DIAP1 decreased radically when Erasp-HA was overexpressed in Drosophila S2 cells (Fig. 5e, f). Despite the limited detection of very small DIAP1 fragments, the cleavage products and amounts of small DIAP1 fragments were higher in Erasp-overexpressing cells than in control cells expressing endogenous levels of Erasp (Fig. 5e). Furthermore, the chase experiment confirmed that the overexpression of Erasp facilitated the degradation of DIAP1, as suggested by the rapid decrease in the levels of DIAP1 compared with that in the control cells expressing endogenous levels of Erasp (Fig. 5g). As the mRNA levels of DIAP1 were comparable in the mock- and Erasp-HA-overexpressing S2 cell lines (Supplementary Fig. 10), we concluded that Erasp actively regulates the expression level of DIAP1 at the posttranslational level.
To determine the domain involved in DIAP1 binding to Erasp, we performed a GST pulldown assay with various DIAP1 truncation mutants fused with GST in Erasp-overexpressing S2 cells. We found that all three domains were required for its interaction with Erasp and that at least two of these domains strengthened the interaction between the two proteins (Fig. 5h). Furthermore, when the catalytically active serine-228 residue in Erasp was mutated, the amount of DIAP1 was significantly restored (Fig. 5i), indicating that Erasp regulates the stability of DIAP1 via its serine protease activity. Finally, the effect of Erasp on DIAP1 degradation was validated using in vivo eye tissues. The results showed that the Rh-1P37H misexpression-induced phenotype (Fig. 5j, m) was evidently suppressed by DIAP1 coexpression (Fig. 5k). In contrast, the eye abnormalities caused by the misexpression of Rh-1P37H were further exacerbated upon Erasp coexpression (Fig. 5l). These findings indicate that the activity of Erasp is elevated under stress conditions, such as ER stress that is induced by misexpression of Rh-1P37H. Moreover, the Rh-1P37H misexpression-driven eye phenotype was suppressed by DIAP1 overexpression (Fig. 5n), and this effect was reversed and aggravated with the coexpression of Erasp (Fig. 5o), confirming that Erasp inhibits the antiapoptotic function of DIAP1. Altogether, these results indicate that Erasp mediates caspase-dependent ER stress-induced apoptosis in Drosophila retinal cells by degrading DIAP1.
Discussion
In the present study, we have reported that Wg/Wnt1 and Erasp were previously unrecognized regulators of ER stress-induced apoptosis in Drosophila. Although several studies have described the Wnt signaling pathway as an essential pathway that regulates growth and is associated with development and cancer, to the best of our knowledge, none of those studies have identified Wnt as a mediator of ER stress-induced cell death. Our data show that the knockdown of Wg/Wnt1 and Erasp, a transcriptional target of Wg/Wnt1, suppressed proapoptotic signaling in a Drosophila model of ADRP. Furthermore, we have demonstrated that Erasp stabilizes Dronc by triggering DIAP1 degradation, thus promoting cell death; therefore, Erasp acts as a mediator of the novel apoptosis signaling pathway that is associated with ER stress-induced cell death (Fig. 6).
The Wnt signaling pathway is highly conserved across various species, from Drosophila and Xenopus to humans, and it controls multiple biological processes during development and adult tissue/organ homeostasis19,44. Wnt proteins, which are secretory glycoproteins, are targeted to the ER and are lipid-modified by Porcupine, an acyl transferase. This lipid modification of Wnt proteins is essential for their secretion as well as their efficient signaling. In the Golgi apparatus, the transmembrane protein Wntless/Evi (Wls) binds to the modified Wnt proteins, facilitating their translocation to the plasma membrane. However, the silencing of Porcupine or Wls leads to defects in Wg/Wnt1 secretion, causing the accumulation of Wg/Wnt1 in the ER and resulting in ER stress59. Accordingly, considering the cellular location of their synthesis and the structural properties of Wnt proteins, it is not surprising that Wnt proteins are involved in ER stress. Our data indicate that ER stress induced by the misexpression of Rh-1P37H activates the Wg/Wnt1 signaling pathway to promote the apoptosis of Drosophila retinal cells. However, the upstream molecular signals that activate the Wg/Wnt1 pathway were not clarified through our experimental system. A recent study has indicated that the expression of Porcupine and Wls increases under various stresses, including ER stress60. Thus, we hypothesized that the increased levels of Porcupine and Wls might promote the secretion of Wnt and thus induce pro-apoptotic gene expression under excess ER stress.
In this study, we propose the function of Erasp, which is classified as a serine protease, as a novel IAP antagonist in Drosophila. The expression of Erasp induces cell death, as evidenced by the appearance of a weak rough eye phenotype upon Erasp misexpression (Fig. 4h, h’). In addition, elevated Erasp expression enhanced caspase activity without apoptotic stimuli, further supporting our hypothesis (Supplementary Fig. 11). Thus, the expression or activity of Erasp should be tightly regulated to allow cell survival. We also hypothesized that Erasp might perform functions similar to those of Drosophila OMI/HTRA2, which is a known mitochondria-localized IAP antagonist57,58. The removal of the mitochondrial targeting sequence in Drosophila OMI/HTRA2 exposes an IAP-binding motif (IBM) that is vital for its interaction with IAP, and the processed Drosophila Omi/HtrA2 then interacts with DIAP1. As the Erasp protein sequence does not contain a mitochondrial targeting sequence (MTS), such as that in Drosophila OMI/HTRA2, it is logical to conclude that Erasp proteins are not likely to be transported to mitochondria. Thus, the activity of Erasp is controlled in quite a different manner from the regulatory mechanism of Drosophila OMI/HTRA2. Considering that the expression of Erasp is regulated by Wg/Wnt1 under conditions of Rh-1P37H misexpression-induced ER stress, the activity of Erasp appears to be modulated via transcriptional activation. However, it is also possible that the activity of Erasp is controlled by posttranslational modification, and this possibility warrants further investigation. Our immunoprecipitation assays suggest that Erasp physically interacts with DIAP1 (Fig. 5c). Through a GST pulldown assay, we were able to identify the binding domains in the two proteins, Erasp and DIAP1. We found that all three domains in DIAP1, namely, the BIR1, BIR2, and RING domains, were required for the interaction between the two proteins and for DIAP1 protein degradation (Fig. 5h). In addition, mutation of the putative residue that confers serine protease activity to Erasp, namely, serine-228, weakened the ability of Erasp to degrade DIAP1 (Fig. 5i). Taken together, our findings suggest that Erasp and Drosophila OMI/HTRA2 are involved in regulating apoptosis, although the mechanism underlying the activation processes of these proteins is quite different.
Our study demonstrates that the knockdown of Wg/Wnt1 delays the course of the retinal degeneration phenotype in the Drosophila model of ADRP. A number of Drosophila Rh-1 allele mutants, widely known as ninaE, serve as faithful models of human ADRP. These endogenous mutants have similar, if not identical, mutations to those found in humans, and they trigger retinal degeneration in an age-dependent manner28,46. Since the cause and pathological outcome of harboring rhodopsin mutations are similar between Drosophila and humans, it appears that the intermediate signaling responses are also conserved between the two species. Similarly, previous studies have shown that aberrant activation of Wnt signaling plays pathological roles in retinal diseases, including diabetic retinopathy and age-related macular degeneration61,62,63. As Wnt proteins also play a central role in various stages of retinal development, we speculate that Wnt signaling may play different roles depending on its environments, such as the developmental stage or cell type.
Similarly, we need to distinguish the apoptotic function of Wnt signaling between apoptosis that is programmed during development and apoptosis that results from ER stress. Wnt is considered to act as a morphogen, which means that its activity is concentration-dependent64. Although Wnt signaling is initiated by the binding of Wnt to its receptor, fz, many extracellular inhibitory components of Wnt signaling, namely, Dickkopf (Dkk), Soluble Frizzled-Related Proteins (SFRPs), and Wnt inhibitory factor (WIF), tightly regulate the binding process via physical interactions or modulation of binding affinity. These extracellular Wnt inhibitors are also known to activate signaling by regulating Wnt stabilization, secretion, or transport, depending on the physiological context18. Moreover, ectopic differences in nuclear components or intracellular components in certain cell types may affect Wnt signaling. Considering all the possible mechanisms by which Wnt signaling is regulated, changes in Wnt levels in cells or tissues that are affected by chronic or extreme ER stress can activate uncontrolled Wnt signaling and ultimately trigger apoptosis via the expression of pro-apoptotic proteins, such as Erasp. To summarize, we suggest that ectopic increases in Wnt expression via the recruitment of different types of Wnt regulatory proteins in certain cells suffering from extreme (chronic) ER stress lead to the activation of pathological Wnt signaling, which may differ from Wnt signaling during normal development. Again, since the p53 overexpression-induced cell death phenotype in the eye does not involve Wg/Wnt1 (Supplementary Fig. 4d,e), we propose that the role of Wg/Wnt1 in cell death is not general but more specific to certain stress-related signaling pathways. Altogether, our study highlights the potential for manipulating Wnt signaling to develop new approaches to therapeutic interventions in ER stress-related diseases.
References
Thompson, C. B. Apoptosis in the pathogenesis and treatment of disease. Science 267, 1456–1462 (1995).
Ron, D. & Walter, P. Signal integration in the endoplasmic reticulum unfolded protein response. Nat. Rev. Mol. Cell. Biol. 8, 519–529 (2007).
Credle, J. J., Finer-Moore, J. S., Papa, F. R., Stroud, R. M. & Walter, P. On the mechanism of sensing unfolded protein in the endoplasmic reticulum. Proc. Natl Acad. Sci. USA 102, 18773–18784 (2005).
Pincus, D. et al. BiP binding to the ER-stress sensor Ire1 tunes the homeostatic behavior of the unfolded protein response. PLoS Biol. 8, e1000415 (2010).
Calfon, M. et al. IRE1 couples endoplasmic reticulum load to secretory capacity by processing the XBP-1 mRNA. Nature 415, 92–96 (2002).
Shen, X. et al. Complementary signaling pathways regulate the unfolded protein response and are required for C. elegans development. Cell 107, 893–903 (2001).
Yoshida, H. et al. Endoplasmic reticulum stress-induced formation of transcription factor complex ERSF including NF-Y (CBF) and activating transcription factors 6alpha and 6beta that activates the mammalian unfolded protein response. Mol. Cell. Biol. 21, 1239–1248 (2001).
Puthalakath, H. et al. ER stress triggers apoptosis by activating BH3-only protein Bim. Cell 129, 1337–1349 (2007).
Timmins, J. M. et al. Calcium/calmodulin-dependent protein kinase II links ER stress with Fas and mitochondrial apoptosis pathways. J Clin. Invest. 119, 2925–2941 (2009).
Wang, Q. et al. ERAD inhibitors integrate ER stress with an epigenetic mechanism to activate BH3-only protein NOXA in cancer cells. Proc. Natl Acad. Sci. USA 106, 2200–2205 (2009).
Hetz, C. et al. Proapoptotic BAX and BAK modulate the unfolded protein response by a direct interaction with IRE1alpha. Science 312, 572–576 (2006).
Marciniak, S. J. et al. CHOP induces death by promoting protein synthesis and oxidation in the stressed endoplasmic reticulum. Genes Dev. 18, 3066–3077 (2004).
Nakagawa, T. et al. Caspase-12 mediates endoplasmic-reticulum-specific apoptosis and cytotoxicity by amyloid-beta. Nature 403, 98–103 (2000).
Nakagawa, T. & Yuan, J. Cross-talk between two cysteine protease families. Activation of caspase-12 by calpain in apoptosis. J. Cell. Biol. 150, 887–894 (2000).
Verfaillie, T. et al. PERK is required at the ER-mitochondrial contact sites to convey apoptosis after ROS-based ER stress. Cell. Death Differ. 19, 1880–1891 (2012).
Li, G., Scull, C., Ozcan, L. & Tabas, I. NADPH oxidase links endoplasmic reticulum stress, oxidative stress, and PKR activation to induce apoptosis. J. Cell. Biol 191, 1113–1125 (2010).
Song, B., Scheuner, D., Ron, D., Pennathur, S. & Kaufman, R. J. Chop deletion reduces oxidative stress, improves beta cell function, and promotes cell survival in multiple mouse models of diabetes. J. Clin. Invest. 118, 3378–3389 (2008).
Clevers, H. Wnt/beta-catenin signaling in development and disease. Cell 127, 469–480 (2006).
Clevers, H. & Nusse, R. Wnt/beta-catenin signaling and disease. Cell 149, 1192–1205 (2012).
Niehrs, C. The complex world of WNT receptor signalling. Nat. Rev. Mol. Cell. Biol. 13, 767–779 (2012).
Ahmed, Y., Hayashi, S., Levine, A. & Wieschaus, E. Regulation of armadillo by a Drosophila APC inhibits neuronal apoptosis during retinal development. Cell 93, 1171–1182 (1998).
Lin, H. V., Rogulja, A. & Cadigan, K. M. Wingless eliminates ommatidia from the edge of the developing eye through activation of apoptosis. Development 131, 2409–2418 (2004).
Lim, H. Y. & Tomlinson, A. Organization of the peripheral fly eye: the roles of Snail family transcription factors in peripheral retinal apoptosis. Development 133, 3529–3537 (2006).
Brand, A. H. & Perrimon, N. Targeted gene expression as a means of altering cell fates and generating dominant phenotypes. Development 118, 401–415 (1993).
Hay, B. A., Wolff, T. & Rubin, G. M. Expression of baculovirus P35 prevents cell death in Drosophila. Development 120, 2121–2129 (1994).
Dietzl, G. et al. A genome-wide transgenic RNAi library for conditional gene inactivation in Drosophila. Nature 448, 151–156 (2007).
Ryoo, H. D., Li, J. & Kang, M. J. Drosophila XBP1 expression reporter marks cells under endoplasmic reticulum stress and with high protein secretory load. PLoS One 8, e75774 (2013).
Colley, N. J., Cassill, J. A., Baker, E. K. & Zuker, C. S. Defective intracellular transport is the molecular basis of rhodopsin-dependent dominant retinal degeneration. Proc. Natl Acad. Sci. USA 92, 3070–3074 (1995).
Pichaud, F. & Desplan, C. A new visualization approach for identifying mutations that affect differentiation and organization of the Drosophila ommatidia. Development 128, 815–826 (2001).
Galy, A., Roux, M. J., Sahel, J. A., Leveillard, T. & Giangrande, A. Rhodopsin maturation defects induce photoreceptor death by apoptosis: a fly model for RhodopsinPro23His human retinitis pigmentosa. Hum. Mol. Genet. 14, 2547–2557 (2005).
Kang, M. J., Chung, J. & Ryoo, H. D. CDK5 and MEKK1 mediate pro-apoptotic signalling following endoplasmic reticulum stress in an autosomal dominant retinitis pigmentosa model. Nat. Cell. Biol. 14, 409–415 (2012).
Kang, K., Ryoo, H. D., Park, J. E., Yoon, J. H. & Kang, M. J. A Drosophila reporter for the translational activation of ATF4 marks stressed cells during development. PLoS One 10, e0126795 (2015).
Tomlinson, A. The cellular dynamics of pattern formation in the eye of Drosophila. J. Embryol. Exp. Morphol. 89, 313–331 (1985).
Kamber Kaya, H. E., Ditzel, M., Meier, P. & Bergmann, A. An inhibitory mono-ubiquitylation of the Drosophila initiator caspase Dronc functions in both apoptotic and non-apoptotic pathways. PLoS Genet 13, e1006438 (2017).
Patel, R. K. & Jain, M. NGS QC Toolkit: a toolkit for quality control of next generation sequencing data. PLoS One 7, e30619 (2012).
Langmead, B., Trapnell, C., Pop, M. & Salzberg, S. L. Ultrafast and memory-efficient alignment of short DNA sequences to the human genome. Genome Biol 10, R25 (2009).
Li, B. & Dewey, C. N. RSEM: accurate transcript quantification from RNA-Seq data with or without a reference genome. BMC Bioinform. 12, 323 (2011).
Love, M. I., Huber, W. & Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol 15, 550 (2014).
Huang da, W., Sherman, B. T. & Lempicki, R. A. Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat. Protoc. 4, 44–57 (2009).
Morishita, J., Kang, M. J., Fidelin, K. & Ryoo, H. D. CDK7 regulates the mitochondrial localization of a tail-anchored proapoptotic protein, Hid. Cell. Rep. 5, 1481–1488 (2013).
Sung, C. H., Schneider, B. G., Agarwal, N., Papermaster, D. S. & Nathans, J. Functional heterogeneity of mutant rhodopsins responsible for autosomal dominant retinitis pigmentosa. Proc. Natl Acad. Sci. USA 88, 8840–8844 (1991).
Mendes, H. F., van der Spuy, J., Chapple, J. P. & Cheetham, M. E. Mechanisms of cell death in rhodopsin retinitis pigmentosa: implications for therapy. Trends Mol. Med. 11, 177–185 (2005).
Ryoo, H. D., Domingos, P. M., Kang, M. J. & Steller, H. Unfolded protein response in a Drosophila model for retinal degeneration. EMBO J. 26, 242–252 (2007).
Nusse, R. & Clevers, H. Wnt/beta-catenin signaling, disease, and emerging therapeutic modalities. Cell 169, 985–999 (2017).
Steinhart, Z. & Angers, S. Wnt signaling in development and tissue homeostasis. Development https://doi.org/10.1242/dev.146589 (2018).
Kurada, P. & O’Tousa, J. E. Retinal degeneration caused by dominant rhodopsin mutations in Drosophila. Neuron 14, 571–579 (1995).
Yanagawa, S., Lee, J. S. & Ishimoto, A. Identification and characterization of a novel line of Drosophila Schneider S2 cells that respond to wingless signaling. J. Biol. Chem. 273, 32353–32359 (1998).
Pang, Y. et al. Structure of the apoptosome: mechanistic insights into activation of an initiator caspase from Drosophila. Genes Dev. 29, 277–287 (2015).
Bao, Q. & Shi, Y. Apoptosome: a platform for the activation of initiator caspases. Cell. Death Differ. 14, 56–65 (2007).
Shapiro, P. J., Hsu, H. H., Jung, H., Robbins, E. S. & Ryoo, H. D. Regulation of the Drosophila apoptosome through feedback inhibition. Nat. Cell Biol. 10, 1440–1446 (2008).
Steller, H. Regulation of apoptosis in Drosophila. Cell. Death Differ. 15, 1132–1138 (2008).
Bergmann, A. The role of ubiquitylation for the control of cell death in Drosophila. Cell. Death Differ. 17, 61–67 (2010).
Hay, B. A. & Guo, M. Caspase-dependent cell death in Drosophila. Annu. Rev. Cell. Dev. Biol. 22, 623–650 (2006).
Wilson, R. et al. The DIAP1 RING finger mediates ubiquitination of Dronc and is indispensable for regulating apoptosis. Nat. Cell. Biol. 4, 445–450 (2002).
Broemer, M. & Meier, P. Ubiquitin-mediated regulation of apoptosis. Trends Cell. Biol. 19, 130–140 (2009).
Ryoo, H. D., Bergmann, A., Gonen, H., Ciechanover, A. & Steller, H. Regulation of Drosophila IAP1 degradation and apoptosis by reaper and ubcD1. Nat. Cell. Biol. 4, 432–438 (2002).
Challa, M. et al. Drosophila Omi, a mitochondrial-localized IAP antagonist and proapoptotic serine protease. EMBO J. 26, 3144–3156 (2007).
Khan, F. S. et al. The interaction of DIAP1 with dOmi/HtrA2 regulates cell death in Drosophila. Cell. Death Differ. 15, 1073–1083 (2008).
Zhang, P., Zhou, L., Pei, C., Lin, X. & Yuan, Z. Dysfunction of Wntless triggers the retrograde Golgi-to-ER transport of Wingless and induces ER stress. Sci. Rep. 6, 19418 (2016).
Mohamed, R., Kennedy, C. & Willmore, W. G. Responses of Porcupine and Wntless proteins to oxidative, hypoxic and endoplasmic reticulum stresses. Cell. Signal. 85, 110047 (2021).
Chen, Y. et al. Activation of the Wnt pathway plays a pathogenic role in diabetic retinopathy in humans and animal models. Am. J. Pathol. 175, 2676–2685 (2009).
Hu, Y. et al. Pathogenic role of the Wnt signaling pathway activation in laser-induced choroidal neovascularization. Invest. Ophthalmol. Vis. Sci. 54, 141–154 (2013).
Tuo, J. et al. Wnt signaling in age-related macular degeneration: human macular tissue and mouse model. J. Transl. Med. 13, 330 (2015).
Logan, C. Y. & Nusse, R. The Wnt signaling pathway in development and disease. Annu. Rev. Cell. Dev. Biol 20, 781–810 (2004).
Acknowledgements
We thank Sun-Cheol Choi and Sang-Wook Kang for their helpful comments on the manuscript and Kyung-Ok Cho, Jean-Paul Vincent, and Shawn B Bratton for the reagents. This work was supported by grants from the National Research Foundation of Korea (NRF 2018R1A2B6003988, 2021R1H1A2093239, and 2022R1A2C1003431), and from the Asan Institute for Life Sciences (Seoul, Republic of Korea; 2019IL0562, 2020IL0002, 2021IL0002, and 2022IL0010).
Author information
Authors and Affiliations
Contributions
J.-E.P. and M.-J.K. designed the experiments. J.-E.P, J.L., S.O., S.B., E.-J.C., and M.-J.K. performed the experiments and analyzed the data. S.-E.Y. and Y.-J.K carried out the generation of the Erasp mutant. J.L. and M.-J.K. wrote the paper, incorporating suggestions from all authors.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Park, JE., Lee, J., Ok, S. et al. Wg/Wnt1 and Erasp link ER stress to proapoptotic signaling in an autosomal dominant retinitis pigmentosa model. Exp Mol Med 55, 1544–1555 (2023). https://doi.org/10.1038/s12276-023-01044-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s12276-023-01044-7